Information
Version: B | 1.1 (2022-01-22)
Please note: This part of the profile is currently being revised.
1 Remarks
1.1 General remarks
Escapees and consequences: negative or at most unpredictable for the local ecosystemPreferences: none in general (i.e. across habitat)
1.2 Other remarks
No data found yet.2 Ethograms
In the farm or lab: on swimming, social behaviour, cognitive abilities, coping styles
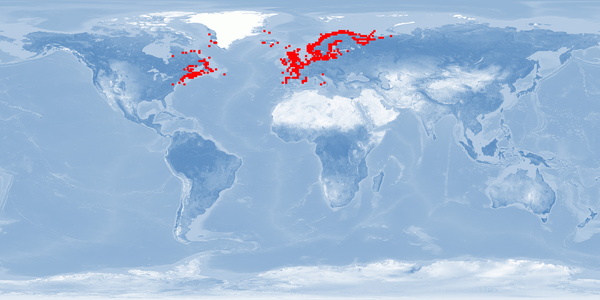
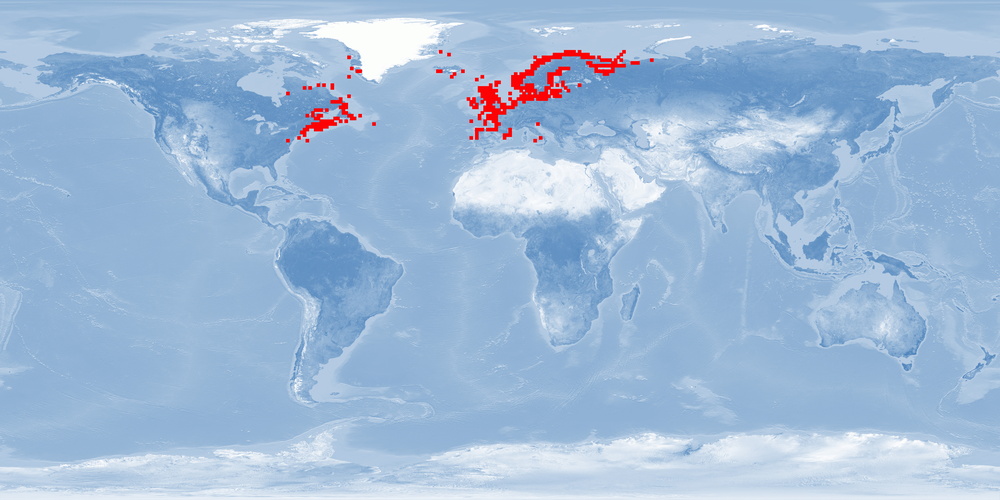
3 Distribution


Natural distribution: northern Atlantic coasts of Canada and USA, southern Greenland, Iceland, United Kingdom, Scandinavia to Portugal, boardering rivers
Introduced: Bering Sea
4 Natural co-existence
5 Substrate and/or shelter
5.1 Substrate
Substrate range, substrate preference: opportunistic – reported from gravel to boulder substrate – but avoids mudSubstrate and growth: direct effect (further research needed)
5.2 Shelter or cover
Shelter or cover preference: rocks and stones for cover from predators or shelter from low or high temperaturesShelter or cover and growth: direct effect (further research needed)
6 Food, foraging, hunting, feeding
6.1 Trophic level and general considerations on food needs
Trophic level: 4.5Impacts of feed fishery: contributes to overfishing, challenges animal welfare
6.2 Food items
Food items, food preference: carnivorous, increasing prey size with increasing age6.3 Feeding behaviour
Feeding style, foraging mode: sit-and-wait but still mobileFeed delivery and stress: unpredicted schedule increases stress and aggression (further research needed)
Food competition and growth: inverse effect (further research needed)
Effects on feeding: direct relation with temperature
For feeding and...
...dominance ➝ D5.
...adaptation to the wild (restocking) ➝ D6,
...exploration-avoidance continuum ➝ D7.
7 Photoperiod
7.1 Daily rhythm
Daily rhythm: diurnal, occasionally nocturnal7.2 Light intensity
No data found yet.7.3 Light colour
No data found yet.8 Water parameters
8.1 Water temperature
Standard temperature range, temperature preference: -0.5-23 °C, 4-17.5 °CTemperature and stress: decreasing survival <2 °C and >22 °C (eggs >16 °C) (further research needed)
Temperature and growth: range 1-26.7 °C, optimally 14-19 °C
8.2 Oxygen
Dissolved oxygen range: ≥7 mg/L (further research needed)8.3 Salinity
Salinity tolerance, standard salinity range: euryhaline8.4 pH
Standard pH range: 6.8-7.9 (further research needed)pH and stress: decreasing survival pH <5.4 (further research needed)
8.5 Turbidity
No data found yet.8.6 Water hardness
No data found yet.8.7 NO4
No data found yet.8.8 Other
No data found yet.9 Swimming
9.1 Swimming type, swimming mode
Swimming type, swimming mode: sub-carangiform9.2 Swimming speed
Swimming speed: 0.2-30 km/d, decreasing activity with decreasing temperaturesStandard velocity range, velocity preference: 1-120 cm/s, 4-50 cm/s, decreasing with decreasing temperatures
9.3 Home range
Home range: 0.005-8+ km, depending on age, barriers to fish passage, availability of suitable habitat9.4 Depth
Depth range, depth preference: 0.05-6.5 m, moves deeper probably to avoid threats9.5 Migration
Migration type: anadromous10 Growth
10.1 Ontogenetic development
Larvae: here called alevins, hatching to ca 300 degree daysFry: beginning of exogenous feeding, ca 35 mm fork length (further research needed)
Juveniles, sexual maturity: fully developed to beginning of maturity, here called parr (first summer after hatching) and smolt (from 2-5 years on)
Maturation and manipulation: advanced photoperiod and ambient temperatures delay maturation (further research needed)
Adults: here called grilse (2-9 years, 50-90 cm, 2-4 kg) and kelt (further research needed)
10.2 Sexual conversion
No data found yet.10.3 Sex ratio
No data found yet.10.4 Effects on growth
Growth and size-grading: mixed effects (further research needed)For growth and...
...substrate ➝ D19,
...shelter or cover ➝ D20,
...food competition ➝ D21,
...temperature ➝ D22,
...stocking density ➝ D23,
...shyness ➝ D24.
10.5 Deformities and malformations
Deformities and malformations: otolith deformations 30-64% worldwide11 Reproduction
11.1 Nest building
Nest building: female cuts redd in <1-100 mm substrate, at 0.2-1.1 m/s water velocity, in 5-76 cm depth11.2 Attraction, courtship, mating
No data found yet.11.3 Spawning
Spawning conditions: gravel, October-January, fresh water, 0.2-1.1 m/s water velocity, 5-76 cm water depth11.4 Fecundity
Female fecundity: average 1 redd with 17-450 eggs11.5 Brood care, breeding
Breeding type: gravel breeder12 Senses
12.1 Vision
Visible spectrum: blue (further research needed)Importance of vision: foraging (further research needed)
12.2 Olfaction (and taste, if present)
Importance of olfaction: probably for navigation (further research needed)12.3 Hearing
Hearing type, hearing spectrum: hearing generalist, 30-380 Hz (further research needed)Noise and stress: sensitive to infrasound (further research needed)
For hearing loss and otolith deformations ➝ D25.
12.4 Touch, mechanical sensing
No data found yet.12.5 Lateral line
Importance of lateral line: probably for navigation (further research needed)12.6 Electrical sensing
No data found yet.12.7 Nociception, pain sensing
No data found yet.12.8 Other
No data found yet.13 Communication
13.1 Visual
No data found yet.13.2 Chemical
No data found yet.13.3 Acoustic
No data found yet.13.4 Mechanical
No data found yet.13.5 Electrical
No data found yet.13.6 Other
No data found yet.14 Social behaviour
14.1 Spatial organisation
Aggregation type: school (further research needed)Stocking density in the wild: 0.06 ind/m2 (further research needed)
Stocking density and stress: direct relation from ca 22 kg/m3 on (further research needed)
Stocking density and growth: mixed effects (further research needed)
14.2 Social organisation
Social organisation type: linear hierarchy (when in small groups)Features of dominance: occupy and patrol best feeding sites, heavier and more aggressive than subordinates
Features of subordination: hardly move, stay away from food
14.3 Exploitation
No data found yet.14.4 Facilitation
No data found yet.14.5 Aggression
Aggression and stocking density: mixed effects (further research needed)Aggression and stress: mixed effects (further research needed)
14.6 Territoriality
Territoriality and feeding: territory decreases with increasing velocity (i.e. increasing food abundance)For territoriality and daily rhythm ➝ D4.
15 Cognitive abilities
15.1 Learning
Classical conditioning: may be used for measuring perceptionAdaptation to wild (restocking): suboptimal with food items and feeding
15.2 Memory
No data found yet.15.3 Problem solving, creativity, planning, intelligence
No data found yet.15.4 Other
No data found yet.16 Personality, coping styles
Exploration-avoidance continuum: relationship with emergence from redd, feeding resumption (further research needed)
Aggressiveness continuum: in dominance-subordination, given stocking density
17 Emotion-like states
17.1 Joy
No data found yet.17.2 Relaxation
No data found yet.17.3 Sadness
No data found yet.17.4 Fear
No data found yet.18 Self-concept, self-recognition
19 Reactions to husbandry
19.1 Stereotypical and vacuum activities
No data found yet.19.2 Acute stress
Confinement: stressful if done for 30 min (further research needed)Crowding: stressful if done at 75 kg/m3 for 60 min, worse if combined with live-chilling (further research needed)
For acute stress and...
...water temperature ➝ D30,
...pH ➝ D31,
...noise ➝ D17,
...shyness ➝ D24,
...stunning ➝ D32.
19.3 Chronic stress
Effects on welfare: cage submergence may be beneficial (further research needed)For chronic stress and...
...cover ➝ D20,
...feed delivery ➝ D33,
...water temperature ➝ D30,
...stocking density ➝ D34,
...aggression ➝ D35.
19.4 Stunning reactions
Stunning rules: fast, effective, safeStunning methods: percussive stunning, spiking most effective (further research needed)
Stunning methods and stress: percussive stunning less stressful than spiking (further research needed)
Glossary
AGGRESSIVENESS = agonistic reactions towards conspecifics. Tests: mirror image, social interaction/diadic encounters 174.
ALEVINS = larvae until the end of yolk sac absorption, for details ➝ Findings 10.1 Ontogenetic development
EXPLORATION-AVOIDANCE = reaction to new situations, e.g. new habitat, new food, novel objects. Referred to as neophobia/neophilia elsewhere. Tests: open field, trappability for first time, novel environment, hole board (time spent with head in holes), novel object 174.
FARM = setting in farming environment or under conditions simulating farming environment in terms of size of facility or number of individuals
FRY = larvae from external feeding on, for details ➝ Findings 10.1 Ontogenetic development
GENERALIST = Generalists detect a narrow bandwidth of sound frequencies (<50-500 Hz, 1,500 Hz max.). High hearing threshold = cannot detect quieter sounds. Typically no swim bladder or no attachment of the swim bladder to the inner ear. Live in loud environments (rivers) 168 169.
GRILSE = adults returning from sea to home river to spawn, for details ➝ Findings 10.1 Ontogenetic development
IND = individuals
JUVENILES = fully developed but immature individuals, for details ➝ Findings 10.1 Ontogenetic development
KELTS = adults surviving spawning, for details ➝ Findings 10.1 Ontogenetic development
LAB = setting in laboratory environment
MILLIARD = 1,000,000,000 73 74
PARR = juvenile stage in rivers, for details ➝ Findings 10.1 Ontogenetic development
PHOTOPERIOD = duration of daylight
SHYNESS-BOLDNESS = reaction to risky (but not new!) situations, e.g. predators or humans. Referred to as docility, tameness, fearfulness elsewhere. Tests: predator presentation, predator stimulus, threat, trappability (latency to enter a trap for first time can be exploration), resistance to handlers (Trapezov stick test), tonic immobility (catatonic-like death-feigning anti predator response) 174.
SMOLTS = juvenile stage migrating to the sea, for details ➝ Findings 10.1 Ontogenetic development
TOTAL LENGTH = from snout to tip of caudal fin as compared to fork length (which measures from snout to fork of caudal fin) 153 or standard length (from head to base of tail fin) or body length (from the base of the eye notch to the posterior end of the telson)
WILD = setting in the wild
Bibliography
2 Brodeur, Richard D., and Morgan S. Busby. 1998. Occurrence of an Atlantic Salmon Salmo salar in the Bering Sea. Alaska Fishery Research Bulletin 5: 64–66.
3 McGinnity, P., C. Stone, J. B. Taggart, D. Cooke, D. Cotter, R. Hynes, C. McCamley, T. Cross, and A. Ferguson. 1997. Genetic impact of escaped farmed Atlantic salmon (Salmo salar L.) on native populations: use of DNA profiling to assess freshwater performance of wild, farmed, and hybrid progeny in a natural river environment. ICES Journal of Marine Science: Journal du Conseil 54: 998–1008. https://doi.org/10.1016/S1054-3139(97)80004-5.
4 Armstrong, J. D, P. S Kemp, G. J. A Kennedy, M Ladle, and N. J Milner. 2003. Habitat requirements of Atlantic salmon and brown trout in rivers and streams. Fisheries Research 62. The Scientific Basis for Management of Salmonid Stocks in the British Isles: 143–170. https://doi.org/10.1016/S0165-7836(02)00160-1.
5 Jacobs, Jürgen. 1974. Quantitative measurement of food selection. Oecologia 14: 413–417. https://doi.org/10.1007/BF00384581.
6 Bremset, Gunnbjørn. 2000. Seasonal and Diel Changes in Behaviour, Microhabitat use and Preferences by Young Pool-dwelling Atlantic Salmon, Salmo salar, and Brown Trout, Salmo trutta. Environmental Biology of Fishes 59: 163–179. https://doi.org/10.1023/A:1007691316864.
7 Bremset, Gunnbjørn, and Ole Kristian Berg. 1999. Three-dimensional microhabitat use by young pool-dwelling Atlantic salmon and brown trout. Animal Behaviour 58: 1047–1059. https://doi.org/10.1006/anbe.1999.1218.
8 Guay, J C, D Boisclair, M Leclerc, and M Lapointe. 2003. Assessment of the transferability of biological habitat models for Atlantic salmon parr (Salmo salar). Canadian Journal of Fisheries and Aquatic Sciences 60: 1398–1408. https://doi.org/10.1139/f03-120.
9 Guay, J C, D Boisclair, D Rioux, M Leclerc, M Lapointe, and P Legendre. 2000. Development and validation of numerical habitat models for juveniles of Atlantic salmon (Salmo salar). Canadian Journal of Fisheries and Aquatic Sciences 57: 2065–2075. https://doi.org/10.1139/f00-162.
10 Coulombe-Pontbriand, M, and Michel Lapointe. 2004. Landscape controls on boulder-rich, winter habitat availability and their effects on Atlantic salmon (Salmo salar) parr abundance in two fifth-order mountain streams. Canadian Journal of Fisheries and Aquatic Sciences 61: 648–658. https://doi.org/10.1139/f04-023.
11 Steingrímsson, Stefán Ó, and James W. A. Grant. 2011. Determinants of multiple central-place territory use in wild young-of-the-year Atlantic salmon Salmo salar. Behavioral Ecology and Sociobiology 65: 275–286. https://doi.org/10.1007/s00265-010-1042-9.
12 Hiscock, M. J., D. A. Scruton, J. A. Brown, and C. J. Pennell. 2002. Diel activity pattern of juvenile Atlantic salmon (Salmo salar ) in early and late winter. Hydrobiologia 483: 161–165. https://doi.org/10.1023/A:1021372822784.
13 Aarestrup, Kim, Christian Nielsen, and Anders Koed. 2002. Net ground speed of downstream migrating radio-tagged Atlantic salmon (Salmo salar L.) and brown trout (Salmo trutta L.) smolts in relation to environmental factors. In Aquatic Telemetry, ed. Eva B. Thorstad, Ian A. Fleming, and Tor Fredrik Næsje, 95–102. Developments in Hydrobiology 165. Springer Netherlands.
14 Crisp, D. T., and P. A. Carling. 1989. Observations on siting, dimensions and structure of salmonid redds. Journal of Fish Biology 34: 119–134. https://doi.org/10.1111/j.1095-8649.1989.tb02962.x.
15 Orlov, Alexander V., Yuri V. Gerasimov, and Oleg M. Lapshin. 2006. The feeding behaviour of cultured and wild Atlantic salmon, Salmo salar L., in the Louvenga River, Kola Peninsula, Russia. ICES J. Mar. Sci. 63: 1297–1303. https://doi.org/10.1016/j.icesjms.2006.05.004.
16 Dill, Peter A. 1977. Development of behaviour in alevins of atlantic salmon, Salmo salar, and rainbow trout, S. gairdneri. Animal Behaviour 25, Part 1: 116–121. https://doi.org/10.1016/0003-3472(77)90073-2.
17 Arnold, G. P., Paul W. Webb, and B. H. Holford. 1991. Short Communication: The Role of the Pectoral Fins in Station-Holding of Atlantic Salmon Parr (Salmo Salar L.). Journal of Experimental Biology 156: 625–629.
18 Armstrong, J. D., and S. W. Griffiths. 2001. Density-dependent refuge use among over-wintering wild Atlantic salmon juveniles. Journal of Fish Biology 58: 1524–1530. https://doi.org/10.1111/j.1095-8649.2001.tb02309.x.
19 Dempster, Tim, Jon-Erik Juell, Jan Erik Fosseidengen, Arne Fredheim, and Pål Lader. 2008. Behaviour and growth of Atlantic salmon (Salmo salar L.) subjected to short-term submergence in commercial scale sea-cages. Aquaculture 276: 103–111. https://doi.org/10.1016/j.aquaculture.2008.01.018.
20 Vaz-Serrano, J., M. L. Ruiz-Gomez, H. M. Gjoen, P. V. Skov, F. A. Huntingford, Øyvind Øverli, and E. Höglund. 2011. Consistent boldness behaviour in early emerging fry of domesticated Atlantic salmon (Salmo salar): Decoupling of behavioural and physiological traits of the proactive stress coping style. Physiology & Behavior 103: 359–364. https://doi.org/10.1016/j.physbeh.2011.02.025.
21 NOT FOUND
22 Valdimarsson, Sveinn K., and Neil B. Metcalfe. 2001. Is the level of aggression and dispersion in territorial fish dependent on light intensity? Animal Behaviour 61: 1143–1149. https://doi.org/10.1006/anbe.2001.1710.
23 Blanchet, S., J. J. Dodson, and S. Brosse. 2006. Influence of habitat structure and fish density on Atlantic salmon Salmo salar L. territorial behaviour. Journal of Fish Biology 68: 951–957. https://doi.org/10.1111/j.0022-1112.2006.00970.x.
24 Adams, C E, J F Turnbull, A Bell, J E Bron, and F A Huntingford. 2007. Multiple determinants of welfare in farmed fish: stocking density, disturbance, and aggression in Atlantic salmon (Salmo salar). Canadian Journal of Fisheries and Aquatic Sciences 64: 336–344. https://doi.org/10.1139/f07-018.
25 Cañon Jones, Hernán Alberto, Chris Noble, Børge Damsgård, and Gareth P. Pearce. 2011. Social network analysis of the behavioural interactions that influence the development of fin damage in Atlantic salmon parr (Salmo salar) held at different stocking densities. Applied Animal Behaviour Science 133: 117–126. https://doi.org/10.1016/j.applanim.2011.05.005.
26 Hawkins, A. D., and A. D. F. Johnstone. 1978. The hearing of the Atlantic Salmon, Salmo salar. Journal of Fish Biology 13: 655–673. https://doi.org/10.1111/j.1095-8649.1978.tb03480.x.
27 Kittilsen, Silje, Tim Ellis, Joachim Schjolden, Bjarne O. Braastad, and Øyvind Øverli. 2009. Determining stress-responsiveness in family groups of Atlantic salmon (Salmo salar) using non-invasive measures. Aquaculture 298: 146–152. https://doi.org/10.1016/j.aquaculture.2009.10.009.
28 Reviewed distribution maps for Atlantic salmon (Salmo salar). 2016. Aquamaps.
29 Netboy, Anthony. 1974. The salmon: their fight for survival: Illustrated with photos. Houghton Mifflin.
30 Elliott, Scott R, Treva A Coe, James M Helfield, and Robert J Naiman. 1998. Spatial variation in environmental characteristics of Atlantic salmon (Salmo salar) rivers. Canadian Journal of Fisheries and Aquatic Sciences 55: 267–280. https://doi.org/10.1139/d98-001.
31 MacCrimmon, Hugh R., and Barra L. Gots. 1979. World Distribution of Atlantic Salmon, Salmo solar. Journal of the Fisheries Research Board of Canada 36: 422–457. https://doi.org/10.1139/f79-062.
32 Crozier, W. W., P.-J. Schön, G. Chaput, E. C. E. Potter, N. Ó Maoiléidigh, and J. C. MacLean. 2004. Managing Atlantic salmon (Salmo salar L.) in the mixed stock environment: challenges and considerations. ICES Journal of Marine Science: Journal du Conseil 61: 1344–1358. https://doi.org/10.1016/j.icesjms.2004.08.013.
33 Jones, M. 2004. Cultured Aquatic Species Information Programme. Salmo salar. Rome: FAO Fisheries and Aquaculture Department.
34 Girard, Isabelle L, James W.A Grant, and Stefán Ó Steingrímsson. 2004. Foraging, growth, and loss rate of young-of-the-year Atlantic salmon (Salmo salar) in relation to habitat use in Catamaran Brook, New Brunswick. Canadian Journal of Fisheries and Aquatic Sciences 61: 2339–2349. https://doi.org/10.1139/f04-216.
35 Roussel, Jean-Marc, Richard A. Cunjak, Robert Newbury, Daniel Caissie, and Alexander Haro. 2004. Movements and habitat use by PIT-tagged Atlantic salmon parr in early winter: the influence of anchor ice. Freshwater Biology 49: 1026–1035. https://doi.org/10.1111/j.1365-2427.2004.01246.x.
36 Cowx, I. G., K. T. O’Grady, W. C. K. O’Connor, and T. E. Andrew. 1998. The effects of siltation on Atlantic salmon, Salmo salar L., embryos in the River Bush. Fisheries Management and Ecology 5: 393–401. https://doi.org/10.1046/j.1365-2400.1998.550393.x.
37 Moir, H. J., C. Soulsby, and A. Youngson. 1998. Hydraulic and sedimentary characteristics of habitat utilized by Atlantic salmon for spawning in the Girnock Burn, Scotland. Fisheries Management and Ecology 5: 241–254. https://doi.org/10.1046/j.1365-2400.1998.00105.x.
38 Soulsby, C., A. F. Youngson, H. J. Moir, and I. A. Malcolm. 2001. Fine sediment influence on salmonid spawning habitat in a lowland agricultural stream: a preliminary assessment. Science of The Total Environment 265: 295–307. https://doi.org/10.1016/S0048-9697(00)00672-0.
39 Davidsen, J. G., N. Plantalech Manel-la, F. Økland, O. H. Diserud, E. B. Thorstad, B. Finstad, R. Sivertsgård, R. S. McKinley, and A. H. Rikardsen. 2008. Changes in swimming depths of Atlantic salmon Salmo salar post-smolts relative to light intensity. Journal of Fish Biology 73: 1065–1074. https://doi.org/10.1111/j.1095-8649.2008.02004.x.
40 Amundsen, Per-Arne, Heidi-Marie Gabler, and Lars Sigvald Riise. 2001. Intraspecific food resource partitioning in Atlantic salmon ( Salmo salar) parr in a subarctic river. Aquatic Living Resources 14: 257–265. https://doi.org/10.1016/S0990-7440(01)01127-5.
41 Erkinaro, J. 1995. The age structure and distribution of Atlantic salmon parr, Salmo salar L., in small tributaries and main stems of the subarctic River Teno, northern Finland. Ecology of Freshwater Fish 4: 53–61. https://doi.org/10.1111/j.1600-0633.1995.tb00117.x.
42 Hesthagen, T. 1990. Home range of juvenile Atlantic salmon, Salmo salar, and brown trout, Salmo trutta, in a Norwegian stream. Freshwater Biology 24: 63–67. https://doi.org/10.1111/j.1365-2427.1990.tb00307.x.
43 Symons, P. E. K., and M. Heland. 1978. Stream Habitats and Behavioral Interactions of Underyearling and Yearling Atlantic Salmon (Salmo salar). Journal of the Fisheries Research Board of Canada 35: 175–183. https://doi.org/10.1139/f78-029.
44 Heggenes, Jan. 1990. Habitat utilization and preferences in juvenile atlantic salmon (salmo salar) in streams. Regulated Rivers: Research & Management 5: 341–354. https://doi.org/10.1002/rrr.3450050406.
45 Heggenes, J., J. L. Baglinière, and R. A. Cunjak. 1999. Spatial niche variability for young Atlantic salmon (Salmo salar) and brown trout (S. trutta) in heterogeneous streams. Ecology of Freshwater Fish 8: 1–21. https://doi.org/10.1111/j.1600-0633.1999.tb00048.x.
46 Beland, K. F., J. G. Trial, and J. F. Kocik. 2004. Use of Riffle and Run Habitats with Aquatic Vegetation by Juvenile Atlantic Salmon. North American Journal of Fisheries Management 24: 525–533. https://doi.org/10.1577/M02-196.1.
47 Amiro, Peter G. 2006. A synthesis of fresh water habitat requirements and status for Atlantic salmon (Salmo salar) in Canada. Research Document 2006/017. Dartmouth, Canada: Canadian Science Advisory Secretariat.
48 Wańkowski, J. W. J., and J. E. Thorpe. 1979. Spatial distribution and feeding in atlantic salmon, Salmo salar L. juveniles. Journal of Fish Biology 14: 239–247. https://doi.org/10.1111/j.1095-8649.1979.tb03515.x.
49 Rimmer, D. M., U. Paim, and R. L. Saunders. 1984. Changes in the Selection of Microhabitat by Juvenile Atlantic Salmon (Salmo salar) at the Summer–Autumn Transition in a Small River. Canadian Journal of Fisheries and Aquatic Sciences 41: 469–475. https://doi.org/10.1139/f84-056.
50 Morantz, D. L., R. K. Sweeney, C. S. Shirvell, and D. A. Longard. 1987. Selection of Microhabitat in Summer by Juvenile Atlantic Salmon (Salmo salar). Canadian Journal of Fisheries and Aquatic Sciences 44: 120–129. https://doi.org/10.1139/f87-015.
51 Adams, J. N., and R. L. Beschta. 1980. Gravel Bed Composition in Oregon Coastal Streams. Canadian Journal of Fisheries and Aquatic Sciences 37: 1514–1521. https://doi.org/10.1139/f80-196.
52 Payne, Brigid A, and Michel F Lapointe. 1997. Channel morphology and lateral stability: effects on distribution of spawning and rearing habitat for Atlantic salmon in a wandering cobble-bed river. Canadian Journal of Fisheries and Aquatic Sciences 54: 2627–2636. https://doi.org/10.1139/f97-171.
53 Julien, H. P., and N. E. Bergeron. 2006. Effect of Fine Sediment Infiltration During the Incubation Period on Atlantic Salmon (Salmo salar) Embryo Survival. Hydrobiologia 563: 61–71. https://doi.org/10.1007/s10750-005-1035-2.
54 Hansen, Tom, and Krisna R. Torrissen. 1985. Artificial hatching substrate and different times of transfer to first feeding: Effect on growth and protease activities of the Atlantic salmon (Salmo salar). Aquaculture 48: 177–188. https://doi.org/10.1016/0044-8486(85)90103-6.
55 Mitchell, J., R. S. McKinley, G. Power, and D. A. Scruton. 1998. Evaluation of Atlantic salmon parr responses to habitat improvement structures in an experimental channel in Newfoundland, Canada. Regulated Rivers: Research & Management 14: 25–39. https://doi.org/10.1002/(SICI)1099-1646(199801/02)14:1<25::AID-RRR474>3.0.CO;2-1.
56 Cunjak, Richard A. 1988. Behaviour and Microhabitat of Young Atlantic Salmon (Salmo salar) during Winter. Canadian Journal of Fisheries and Aquatic Sciences 45: 2156–2160. https://doi.org/10.1139/f88-250.
57 Heggenes, J., Å. Brabrand, and S. J. Saltveit. 1991. Microhabitat use by brown trout, Salmo trutta L. and Atlantic salmon, S. salar L., in a stream: a comparative study of underwater and river bank observations. Journal of Fish Biology 38: 259–266. https://doi.org/10.1111/j.1095-8649.1991.tb03112.x.
58 Valdimarsson, S. K., and N. B. Metcalfe. 1998. Shelter selection in juvenile Atlantic salmon, or why do salmon seek shelter in winter? Journal of Fish Biology 52: 42–49. https://doi.org/10.1111/j.1095-8649.1998.tb01551.x.
59 Bardonnet, Agnès, and Jean-Luc Baglinière. 2000. Freshwater habitat of Atlantic salmon (Salmo salar). Canadian Journal of Fisheries and Aquatic Sciences 57: 497–506. https://doi.org/10.1139/f99-226.
60 Lindberg, Dan-Erik. 2011. Atlantic salmon (Salmo salar) migration behavior and preferences in smolts, spawners and kelts. Report 14. Umeå Swedish University of Agricultural Sciences: Department of Wildlife, Fish and Environmental Studies.
61 Gibson, R. John. 1978. The Behavior of Juvenile Atlantic Salmon (Salmo salar) and Brook Trout (Salvelinus fontinalis) with Regard to Temperature and to Water Velocity. Transactions of the American Fisheries Society 107: 703–712. https://doi.org/10.1577/1548-8659(1978)107<703:TBOJAS>2.0.CO;2.
62 Kottelat, Maurice, and Jörg Freyhof. 2007. Handbook of European freshwater fishes. Publications Kottelat.
63 Froese, R., and D. Pauly. 2014. FishBase. World Wide Web electronic publication. www.fishbase.org.
64 Finstad, A. G., S. Einum, T. Forseth, and O. Ugedal. 2007. Shelter availability affects behaviour, size-dependent and mean growth of juvenile Atlantic salmon. Freshwater Biology 52: 1710–1718. https://doi.org/10.1111/j.1365-2427.2007.01799.x.
65 Gries, Gabe, and Francis Juanes. 1998. Microhabitat use by juvenile Atlantic salmon (Salmo salar ) sheltering during the day in summer. Canadian Journal of Zoology 76: 1441–1449. https://doi.org/10.1139/z98-074.
66 Holbrook, Christopher M., Joseph Zydlewski, Dimitry Gorsky, Steven L. Shepard, and Michael T. Kinnison. 2009. Movements of Prespawn Adult Atlantic Salmon Near Hydroelectric Dams in the Lower Penobscot River, Maine. North American Journal of Fisheries Management 29: 495–505. https://doi.org/10.1577/M08-042.1.
67 Breau, Cindy, Richard A. Cunjak, and Stephan J. Peake. 2011. Behaviour during elevated water temperatures: can physiology explain movement of juvenile Atlantic salmon to cool water? Journal of Animal Ecology 80: 844–853. https://doi.org/10.1111/j.1365-2656.2011.01828.x.
68 Pickering, A. D., R. Griffiths, and T. G. Pottinger. 1987. A comparison of the effects of overhead cover on the growth, survival and haematology of juvenile Atlantic salmon, Salmo salar L., brown trout, Salmo trutta L., and rainbow trout, Salmo gairdneri Richardson. Aquaculture 66: 109–124. https://doi.org/10.1016/0044-8486(87)90226-2.
69 FAO. 2014. The State of World Fisheries and Aquaculture 2014. Rome: Food and Agriculture Organization of the United Nations.
70 Watson, R., Jackie Alder, and Daniel Pauly. 2006. Fisheries for forage fish, 1950 to the present. In On the Multiple Uses of Forage Fish: from Ecosystems to Markets, ed. Jackie Alder and Daniel Pauly, 14:1–20. Fisheries Centre Research Reports 3. Vancouver, Canada: Fisheries Centre, University of British Columbia.
71 Mood, A. 2012. Average annual fish capture for species mostly used for fishmeal (2005-2009). fishcount.org.uk.
72 Mood, A., and P. Brooke. 2012. Estimating the Number of Farmed Fish Killed in Global Aquaculture Each Year.
73 Kopf, Von Kristin. 2012. Milliarden vs. Billionen: Große Zahlen. Sprachlog.
74 Weisstein, Eric W. 2018. Milliard. Text. MathWorld - a Wolfram Web resource. http://mathworld.wolfram.com/Milliard.html. Accessed February 2.
75 Gardiner, W. Ross, and Peter Geddes. 1980. The influence of body composition on the survival of juvenile salmon. Hydrobiologia 69: 67–72. https://doi.org/10.1007/BF00016537.
76 Elliott, J. M., and M. A. Hurley. 1997. A functional model for maximum growth of Atlantic Salmon parr, Salmo salar, from two populations in northwest England. Functional Ecology 11: 592–603. https://doi.org/10.1046/j.1365-2435.1997.00130.x.
77 Finstad, Anders G., Tor F. Næsje, and Torbjørn Forseth. 2004. Seasonal variation in the thermal performance of juvenile Atlantic salmon (Salmo salar). Freshwater Biology 49: 1459–1467. https://doi.org/10.1111/j.1365-2427.2004.01279.x.
78 Elliott, J. M., and J. A. Elliott. 2010. Temperature requirements of Atlantic salmon Salmo salar, brown trout Salmo trutta and Arctic charr Salvelinus alpinus: predicting the effects of climate change. Journal of Fish Biology 77: 1793–1817. https://doi.org/10.1111/j.1095-8649.2010.02762.x.
79 Fraser, Neil H. C., Niel B. Metcalfe, and John E. Thorpe. 1993. Temperature-Dependent Switch between Diurnal and Nocturnal Foraging in Salmon. Proceedings of the Royal Society of London B: Biological Sciences 252: 135–139. https://doi.org/10.1098/rspb.1993.0057.
80 Hoar, William S. 1942. Diurnal Variations in Feeding Activity of Young Salmon and Trout. Journal of the Fisheries Research Board of Canada 6a: 90–101. https://doi.org/10.1139/f42-011.
81 Hirata, Hachiro. 1973. Diurnal Rhythm of Metabolic and Activity Rates in Juvenile Atlantic Salmon, Salmo salar LINNAEUS. Memoirs of Faculty of Fisheries Kagoshima University 22: 73–93.
82 Fraser, Neil H. C., Neil B. Metcalfe, Jan Heggenes, and John E. Thorpe. 1995. Low summer temperatures cause juvenile Atlantic salmon to become nocturnal. Canadian Journal of Zoology 73: 446–451. https://doi.org/10.1139/z95-051.
83 Moore, A., E. C. E. Potter, N. J. Milner, and S. Bamber. 1995. The migratory behaviour of wild Atlantic salmon (Salmo salar) smolts in the estuary of the River Conwy, North Wales. Canadian Journal of Fisheries and Aquatic Sciences 52: 1923–1935. https://doi.org/10.1139/f95-784.
84 Lacroix, Gilles L., Paul McCurdy, and Derek Knox. 2004. Migration of Atlantic Salmon Postsmolts in Relation to Habitat Use in a Coastal System. Transactions of the American Fisheries Society 133: 1455–1471. https://doi.org/10.1577/T03-032.1.
85 Woodhead, P. M. J. 1957. Reactions of Salmonid Larvae to Light. Journal of Experimental Biology 34: 402–416.
86 Jobling, M. 1981. Temperature tolerance and the final preferendum—rapid methods for the assessment of optimum growth temperatures. Journal of Fish Biology 19: 439–455. https://doi.org/10.1111/j.1095-8649.1981.tb05847.x.
87 Johansson, David, Kari Ruohonen, Jon-Erik Juell, and Frode Oppedal. 2009. Swimming depth and thermal history of individual Atlantic salmon (Salmo salar L.) in production cages under different ambient temperature conditions. Aquaculture 290: 296–303. https://doi.org/10.1016/j.aquaculture.2009.02.022.
88 Johansson, David, Kari Ruohonen, Anders Kiessling, Frode Oppedal, Jan-Erik Stiansen, Mark Kelly, and Jon-Erik Juell. 2006. Effect of environmental factors on swimming depth preferences of Atlantic salmon (Salmo salar L.) and temporal and spatial variations in oxygen levels in sea cages at a fjord site. Aquaculture 254: 594–605. https://doi.org/10.1016/j.aquaculture.2005.10.029.
89 Forsythe, Michael George. 1968. Analysis of the 1966 smolt run in the Northwest Miramichi River, New Brunswick. Technical Report 91. Ottawa: Fisheries Research Board of Canada.
90 Crisp, D. T. 1996. Environmental requirements of common riverine European salmonid fish species in fresh water with particular reference to physical and chemical aspects. Hydrobiologia 323: 201–221. https://doi.org/10.1007/BF00007847.
91 Chadwick, E. M. P. 1982. Stock-Recruitment Relationship for Atlantic Salmon (Salmo salar) in Newfoundland Rivers. Canadian Journal of Fisheries and Aquatic Sciences 39: 1496–1501. https://doi.org/10.1139/f82-201.
92 Cunjak, R. A., and J. Therrien. 1998. Inter-stage survival of wild juvenile Atlantic salmon, Salmo salar L. Fisheries Management and Ecology 5: 209–223. https://doi.org/10.1046/j.1365-2400.1998.00094.x.
93 Baglinière, Jean Luc, Gérard Maisse, and Alix Nihouarn. 1993. Comparison of two methods of Atlantic salmon (Salmo salar) wild smolt production. In Production of Juvenile Atlantic Salmon, Salmo Salar, in Natural Waters, ed. R. John Gibson and Richard E. Cutting, 189–201. Canadian Journal of Fisheries and Aquatic Sciences 118. NRC Research Press.
94 Cunjak, R A. 1996. Winter habitat of selected stream fishes and potential impacts from land-use activity. Canadian Journal of Fisheries and Aquatic Sciences 53: 267–282. https://doi.org/10.1139/f95-275.
95 Symons, Philip E. K. 1979. Estimated Escapement of Atlantic Salmon (Salmo salar) for Maximum Smolt Production in Rivers of Different Productivity. Journal of the Fisheries Research Board of Canada 36: 132–140. https://doi.org/10.1139/f79-022.
96 Kennedy, G. J. A, and W. W. Crozier. 1993. Juvenile Atlantic Salmon (Salmo salar) - Production and Prediction. In Production of Juvenile Atlantic Salmon, Salmo Salar, in Natural Waters, ed. R. John Gibson and Richard E. Cutting, 179–187. Canadian Special Publication of Fisheries and Aquatic Sciences 118. NRC Research Press.
97 Gibson, R. John. 1995. Regulation of the fitness of altantic salmon ( Salmo salar ) by intra-specific competition amongst the juveniles. Freshwater Forum A Journal of the Freshwater Biological Association 5: 54–72.
98 Lee, R. L.G., and G. Power. 1976. Atlantic Salmon (Salmo salar) of the Leaf River, Ungava Bay. Journal of the Fisheries Research Board of Canada 33: 2616–2621. https://doi.org/10.1139/f76-311.
99 Power, Geoffrey. 1969. The Salmon of Ungava Bay. Arctic Institute of North America.
100 Jensen, Arne Johan, and Bjørn Ove Johnsen. 1986. Different Adaptation Strategies of Atlantic Salmon (Salmo salar) Populations to Extreme Climates with Special Reference to some Cold Norwegian Rivers. Canadian Journal of Fisheries and Aquatic Sciences 43: 980–984. https://doi.org/10.1139/f86-120.
101 Jensen, Arne J., Bjørn O. Johnsen, and Laila Saksgård. 2011. Temperature Requirements in Atlantic Salmon (Salmo salar), Brown Trout (Salmo trutta), and Arctic Char (Salvelinus alpinus) from Hatching to Initial Feeding Compared with Geographic Distribution. Canadian Journal of Fisheries and Aquatic Sciences. Ottawa, Canada. https://doi.org/10.1139/f89-097.
102 Evans, Geoffrey T., Jake C. Rice, and E. Michael P. Chadwick. 1985. Patterns of Growth and Smolting of Atlantic Salmon (Salmo salar) Parr in a Southwestern Newfoundland River. Canadian Journal of Fisheries and Aquatic Sciences 42: 539–543. https://doi.org/10.1139/f85-071.
103 Allen, K. R. 1969. Limitations on production in salmonid populations in streams. In Symposium on Salmon and Trout in Streams, ed. T. G. Northcote, 3–18. Institute of Fisheries, University of British Columbia.
104 Higgins, P. J., and C. Talbot. 1985. Growth and feeding in juvenile Atlantic salmon (Salmo salar L.). In Nutrition and Feeding in Fish, ed. C. B. Cowey, Alexander Milne Mackie, and J. G. Bell, 243–263. Acad. Press.
105 Dwyer, William P., and Robert G. Piper. 1987. Atlantic Salmon Growth Efficiency as Affected by Temperature. The Progressive Fish-Culturist 49: 57–59. https://doi.org/10.1577/1548-8640(1987)49<57:ASGEAA>2.0.CO;2.
106 Peterson, R. H., and D. J. Martin-Robichaud. 1989. First feeding of Atlantic salmon (Salmo salar L.) fry as influenced by temperature regime. Aquaculture 78: 35–53. https://doi.org/10.1016/0044-8486(89)90004-5.
107 Webb, Paul W. 1984. Form and Function in Fish Swimming. Scientific American 251: 72–83.
108 Wootton, Robert J. 1990. Ecology of Teleost Fishes. London: Chapman and Hall.
109 Katopodis, Chris. 1992. Introduction to fishway design.
110 Fänge, R. 1952. The mechanisms of gas transport in the euphysoclist swimbladder. Acta physiologica Scandinavica. Supplementum 30: 1–133.
111 Bui, Samantha, Frode Oppedal, Øyvind J. Korsøen, and Tim Dempster. 2013. Modifying Atlantic salmon behaviour with light or feed stimuli may improve parasite control techniques. Aquaculture Environment Interactions 3: 125–133. https://doi.org/10.3354/aei00055.
112 Furevik, Dag M., Åsmund Bjordal, Ingvar Huse, and Anders Fernö. 1993. Surface activity of Atlantic salmon (Salmo salar L.) in net pens. Aquaculture 110: 119–128. https://doi.org/10.1016/0044-8486(93)90266-2.
113 NOT FOUND
114 Rivinoja, P., J. Östergren, K. Leonardsson, H. Lundqvist, J. Kiviloog, L. Bergdah, and L. Brydsten. 2004. Downstream migration of Salmo salar and S. trutta smolts in two regulated northern Swedish rivers.
115 Holm, M., J. Chr Holst, and L. P. Hansen. 2000. Spatial and temporal distribution of post-smolts of Atlantic salmon (Salmo salar L.) in the Norwegian Sea and adjacent areas. ICES Journal of Marine Science: Journal du Conseil 57: 955–964. https://doi.org/10.1006/jmsc.2000.0700.
116 Dadswell, M. J., A. D. Spares, J. M. Reader, and M. J. W. Stokesbury. 2010. The North Atlantic subpolar gyre and the marine migration of Atlantic salmon Salmo salar: the ‘Merry-Go-Round’ hypothesis. Journal of Fish Biology 77: 435–467. https://doi.org/10.1111/j.1095-8649.2010.02673.x.
117 Enders, Eva C., Michael H. Gessel, and John G. Williams. 2009. Development of successful fish passage structures for downstream migrants requires knowledge of their behavioural response to accelerating flow. Canadian Journal of Fisheries and Aquatic Sciences 66: 2109–2117. https://doi.org/10.1139/F09-141.
118 Haro, Alex, Mufeed Odeh, John Noreika, and Theodore Castro-Santos. 1998. Effect of Water Acceleration on Downstream Migratory Behavior and Passage of Atlantic Salmon Smolts and Juvenile American Shad at Surface Bypasses. Transactions of the American Fisheries Society 127: 118–127. https://doi.org/10.1577/1548-8659(1998)127<0118:EOWAOD>2.0.CO;2.
119 Ruggles, C. P. 1980. A Review of the Downstream Migration of Atlantic Salmon. Vol. 952. Canadian Technical Report of Fisheries and Aquatic Sciences. Fisheries and Oceans Canada.
120 Hubley, P. Bradford, Peter G. Amiro, A. Jamie F. Gibson, Gilles L. Lacroix, and Anna M. Redden. 2008. Survival and behaviour of migrating Atlantic salmon (Salmo salar L.) kelts in river, estuarine, and coastal habitat. ICES Journal of Marine Science: Journal du Conseil 65: 1626–1634. https://doi.org/10.1093/icesjms/fsn129.
121 Davidsen, J. G., A. H. Rikardsen, E. Halttunen, E. B. Thorstad, F. Økland, B. H. Letcher, J. Skarðhamar, and T. F. Næsje. 2009. Migratory behaviour and survival rates of wild northern Atlantic salmon Salmo salar post-smolts: effects of environmental factors. Journal of Fish Biology 75: 1700–1718. https://doi.org/10.1111/j.1095-8649.2009.02423.x.
122 Halttunen, Elina, Audun H. Rikardsen, Jan G. Davidsen, Eva B. Thorstad, and J. Brian Dempson. 2009. Survival, Migration Speed and Swimming Depth of Atlantic Salmon Kelts During Sea Entry and Fjord Migration. In Tagging and Tracking of Marine Animals with Electronic Devices, ed. Jennifer L. Nielsen, Haritz Arrizabalaga, Nuno Fragoso, Alistair Hobday, Molly Lutcavage, and John Sibert, 35–49. Reviews: Methods and Technologies in Fish Biology and Fisheries 9. Springer Netherlands.
123 Rimmer, D. M., R. L. Saunders, and U. Paim. 1985. Effects of temperature and season on the position holding performance of juvenile Atlantic salmon (Salmo salar). Canadian Journal of Zoology 63: 92–96. https://doi.org/10.1139/z85-017.
124 Rimmer, D. M., and U. Paim. 1990. Effects of temperature, photoperiod, and season on the photobehaviour of juvenile Atlantic salmon (Salmo salar). Canadian Journal of Zoology 68: 1098–1103. https://doi.org/10.1139/z90-162.
125 Graham, W Douglas, John E Thorpe, and Neil B Metcalfe. 1996. Seasonal current holding performance of juvenile Atlantic salmon in relation to temperature and smolting. Canadian Journal of Fisheries and Aquatic Sciences 53: 80–86. https://doi.org/10.1139/f95-167.
126 Gibson, R. John. 1988. Mechanisms regulating species composition, population structure, and production of stream salmonids; a review. Polish Archives of Hydrobiology 35: 469–495.
127 Salinger, David H., and James J. Anderson. 2006. Effects of Water Temperature and Flow on Adult Salmon Migration Swim Speed and Delay. Transactions of the American Fisheries Society 135: 188–199. https://doi.org/10.1577/T04-181.1.
128 Degraaf, D. A., and L. H. Bain. 1986. Habitat Use by and Preferences of Juvenile Atlantic Salmon in Two Newfoundland Rivers. Transactions of the American Fisheries Society 115: 671–681. https://doi.org/10.1577/1548-8659(1986)115<671:HUBAPO>2.0.CO;2.
129 Crisp, D. Trevor. 1993. The environmental requirements of salmon and trout in fresh water. Freshwater Forum 3: 176–202.
130 Bult, Tammo P, Stephen C Riley, Richard L Haedrich, R John Gibson, and Jan Heggenes. 1999. Density-dependent habitat selection by juvenile Atlantic salmon (Salmo salar) in experimental riverine habitats. Canadian Journal of Fisheries and Aquatic Sciences 56: 1298–1306. https://doi.org/10.1139/f99-074.
131 Holm, C. F., J. D. Armstrong, and D. J. Gilvear. 2001. Investigating a major assumption of predictive instream habitat models: is water velocity preference of juvenile Atlantic salmon independent of discharge? Journal of Fish Biology 59: 1653–1666. https://doi.org/10.1111/j.1095-8649.2001.tb00228.x.
132 Metcalfe, Neil B., Sveinn K. Valdimarsson, and Neil H. C. Fraser. 1997. Habitat Profitability and Choice in a Sit-And-Wait Predator: Juvenile Salmon Prefer Slower Currents on Darker Nights. Journal of Animal Ecology 66: 866–875. https://doi.org/10.2307/6002.
133 Kallio-Nyberg, Irma, Heikki Peltonen, and Hannu Rita. 1999. Effects of stock-specific and environmental factors on the feeding migration of Atlantic salmon (Salmo salar) in the Baltic Sea. Canadian Journal of Fisheries and Aquatic Sciences 56: 853–861. https://doi.org/10.1139/f99-022.
134 Kennedy, G. J. A., and C. D. Strange. 1982. The distribution of salmonids in upland streams in relation to depth and gradient. Journal of Fish Biology 20: 579–591. https://doi.org/10.1111/j.1095-8649.1982.tb03956.x.
135 Døving, Kjell B., Håkan Westerberg, and Peter B. Johnsen. 1985. Role of Olfaction in the Behavioral and Neuronal Responses of Atlantic Salmon, Salmo salar, to Hydrographic Stratification. Canadian Journal of Fisheries and Aquatic Sciences 42: 1658–1667. https://doi.org/10.1139/f85-207.
136 Mäki-Petäys, Aki, Ari Huusko, Jaakko Erkinaro, and Timo Muotka. 2002. Transferability of habitat suitability criteria of juvenile Atlantic salmon (Salmo salar). Canadian Journal of Fisheries and Aquatic Sciences 59: 218–228. https://doi.org/10.1139/f01-209.
137 Thorpe, J. E., and R. I. G. Morgan. 1978. Periodicity in Atlantic salmon Salmo salar L. smolt migration. Journal of Fish Biology 12: 541–548. https://doi.org/10.1111/j.1095-8649.1978.tb04200.x.
138 Youngson, A. F., R. J. G. Buck, T. H. Simpson, and D. W. Hay. 1983. The autumn and spring emigrations of juvenile Atlantic salmon, Salmo salar L., from the Girnock Burn, Aberdeenshire, Scotland: environmental release of migration. Journal of Fish Biology 23: 625–639. https://doi.org/10.1111/j.1095-8649.1983.tb02942.x.
139 Hansen, Lars P., and Bror Jonsson. 1985. Downstream migration of hatchery-reared smolts of Atlantic salmon (Salmo salar L.) in the River Imsa, Norway. Aquaculture 45. Salmonid Smoltification II: 237–248. https://doi.org/10.1016/0044-8486(85)90273-X.
140 Hesthagen, Trygve, and Erik Garnås. 1986. Migration of Atlantic Salmon Smolts in River Orkla of Central Norway in Relation to Management of a Hydroelectric Station. North American Journal of Fisheries Management 6: 376–382. https://doi.org/10.1577/1548-8659(1986)6<376:MOASSI>2.0.CO;2.
141 Solomon, D. J. 1982. Smolt migration in Atlantic salmon (Salmo salar L.) and sea trout (Salmo trutta L.). In Proceedings of the Salmon and Trout Migratory Behavior Symposium - International Symposium, ed. E. L. Brannon and E. O. Salo, 196–203. Seattle: University of Washington.
142 Jutila, E., E. Jokikokko, and E. Ikonen. 2009. Post-smolt migration of Atlantic salmon, Salmo salar L., from the Simojoki river to the Baltic Sea. Journal of Applied Ichthyology 25: 190–194. https://doi.org/10.1111/j.1439-0426.2009.01212.x.
143 Davidsen, Jan, Martin-A. Svenning, Panu Orell, Nigel Yoccoz, J. Brian Dempson, Eero Niemelä, Anders Klemetsen, Anders Lamberg, and Jaakko Erkinaro. 2005. Spatial and temporal migration of wild Atlantic salmon smolts determined from a video camera array in the sub-Arctic River Tana. Fisheries Research 74: 210–222. https://doi.org/10.1016/j.fishres.2005.02.005.
144 Mork, O. I., and J. Gulbrandsen. 1994. Vertical activity of four salmonid species in response to changes between darkness and two intensities of light. Aquaculture 127: 317–328. https://doi.org/10.1016/0044-8486(94)90234-8.
145 Bratland, Silje, Lars Helge Stien, Victoria A. Braithwaite, Jon-Erik Juell, Ole Folkedal, Jonatan Nilsson, Frode Oppedal, Jan Erik Fosseidengen, and Tore S. Kristiansen. 2010. From fright to anticipation: using aversive light stimuli to investigate reward conditioning in large groups of Atlantic salmon (Salmo salar). Aquaculture International 18: 991–1001. https://doi.org/10.1007/s10499-009-9317-8.
146 Folkedal, O., T. Torgersen, J. Nilsson, and F. Oppedal. 2010. Habituation rate and capacity of Atlantic salmon (Salmo salar) parr to sudden transitions from darkness to light. Aquaculture 307: 170–172. https://doi.org/10.1016/j.aquaculture.2010.06.001.
147 Johnsson, Jörgen I, Johan Höjesjö, and Ian A Fleming. 2001. Behavioural and heart rate responses to predation risk in wild and domesticated Atlantic salmon. Canadian Journal of Fisheries and Aquatic Sciences 58: 788–794. https://doi.org/10.1139/f01-025.
148 Balon, Eugene K. 1990. Epigenesis of an epigeneticist: the development of some alternative concepts on the early ontogeny and evolution of fishes. Guelph Ichthyology Reviews 1: 1–48.
149 Cunjak, Richard A. 1995. Adressing Forestry Impacts in the Catamaran Brook Basin: An Overview of the Prelogging Phase. In Water, Science, and the Public: The Miramichi Ecosystem, ed. Edward Michael Pakenham Chadwick, 191–210. Canadian Special Publication of Fisheries and Aquatic Sciences 123. NRC Research Press.
150 FAO. 2014. FAO Fisheries & Aquaculture - Species Fact Sheets - Salmo salar (Linnaeus, 1758). World Wide Web electronic publication. www.fao.org.
151 Armstrong, John D, James WA Grant, Harvey L Forsgren, Kurt D Fausch, Richard M DeGraaf, Ian A Fleming, Terry D Prowse, and Isaac J Schlosser. 1998. The application of science to the management of Atlantic salmon (Salmo salar): integration across scales. Canadian Journal of Fisheries and Aquatic Sciences 55: 303–311. https://doi.org/10.1139/d98-014.
152 Cunjak, Richard A., Daniel Caissie, N. El-Jabi, P. Hardie, J. H. Conlon, T. L. Pollock, D. J. Giberson, and S. Komadina-Douthwright. 1993. The Catamaran Brook (New Brunswick) habitat research project: biological, physical and chemical conditions (1990-1992). Canadian Technical Report of Fisheries and Aquatic Sciences 1914. Moncton, NB: Dept. of Fisheries and Oceans, Gulf Region, Science Branch, Fish Habitat and Enhancement Division.
153 Pawson, M.G., and G.D. Pickett. 1996. The Annual Pattern of Condition and Maturity in Bass, Dicentrarchus Labrax, in Waters Around England and Wales. Journal of the Marine Biological Association of the United Kingdom 76: 107. https://doi.org/10.1017/S0025315400029040.
154 Adams, Colin E., and John E. Thorpe. 1989. Photoperiod and temperature effects on early development and reproductive investment in Atlantic salmon (Salmo salar L.). Aquaculture 79: 403–409. https://doi.org/10.1016/0044-8486(89)90483-3.
155 Skjervold, Per Olav, Svein Olav Fjæra, Per Braarød Østby, and Olai Einen. 2001. Live-chilling and crowding stress before slaughter of Atlantic salmon (Salmo salar). Aquaculture 192: 265–280. https://doi.org/10.1016/S0044-8486(00)00447-6.
156 NOT FOUND
157 Reimer, T., T. Dempster, F. Warren-Myers, A. J. Jensen, and S. E. Swearer. 2016. High prevalence of vaterite in sagittal otoliths causes hearing impairment in farmed fish. Scientific Reports 6. https://doi.org/10.1038/srep25249.
158 Warner, Kendall. 1963. Natural Spawning Success of Landlocked Salmon, Salmo salar. Transactions of the American Fisheries Society 92: 161–164. https://doi.org/10.1577/1548-8659(1963)92[161:NSSOLS]2.0.CO;2.
159 Kondolf, G. Mathias, and M. Gordon Wolman. 1993. The sizes of salmonid spawning gravels. Water Resources Research 29: 2275–2285. https://doi.org/10.1029/93WR00402.
160 Heggberget, T. G. 1991. Some Environmental Requirements of Atlantic Salmon. In Fisheries Bioengineering Symposium: American Fisheries Society Symposium, ed. J. Colt and R. J. White, 10:132–135. Bethesda, MD: American Fisheries Society.
161 Beland, Kenneth F., Richard M. Jordan, and Alfred L. Meister. 1982. Water Depth and Velocity Preferences of Spawning Atlantic Salmon in Maine Rivers. North American Journal of Fisheries Management 2: 11–13. https://doi.org/10.1577/1548-8659(1982)2<11:WDAVPO>2.0.CO;2.
162 Walker, Michael M., Carol E. Diebel, Cordula V. Haugh, Patricia M. Pankhurst, John C. Montgomery, and Colin R. Green. 1997. Structure and function of the vertebrate magnetic sense. Nature 390: 371–376. https://doi.org/10.1038/37057.
163 Moore, A., S. M. Freake, and I. M. Thomas. 1990. Magnetic Particles in the Lateral Line of the Atlantic Salmon (Salmo salar L.). Philosophical Transactions of the Royal Society of London B: Biological Sciences 329: 11–15. https://doi.org/10.1098/rstb.1990.0145.
164 Nordeng, Hans. 1971. Is the Local Orientation of Anadromous Fishes determined by Pheromones ? Nature 233: 411–413. https://doi.org/10.1038/233411a0.
165 Leggett, W C. 1977. The Ecology of Fish Migrations. Annual Review of Ecology and Systematics 8: 285–308. https://doi.org/10.1146/annurev.es.08.110177.001441.
166 Hansen, L P, and T P Quinn. 1998. The marine phase of the Atlantic salmon (Salmo salar) life cycle, with comparisons to Pacific salmon. Canadian Journal of Fisheries and Aquatic Sciences 55: 104–118. https://doi.org/10.1139/d98-010.
167 Gorsky, Dimitry, Joan Trial, Joseph Zydlewski, and James McCleave. 2009. The Effects of Smolt Stocking Strategies on Migratory Path Selection of Adult Atlantic Salmon in the Penobscot River, Maine. North American Journal of Fisheries Management 29: 949–957. https://doi.org/10.1577/M08-068.1.
168 Brown, Culum. 2015. Fish intelligence, sentience and ethics. Animal Cognition 18: 1–17. https://doi.org/10.1007/s10071-014-0761-0.
169 Amundsen, Lasse, and Martin Landro. 2011. Marine seismic sources part VIII: Fish hear a great deal. Recent Advances in Technology 8: 1–5.
170 Dijkgraaf, S. 1967. Biological significance of the lateral line organs. In Lateral Line Detectors: Proceedings. Edited by Phyllis H. Cahn, ed. Phyllis H. Cahn, 83–95. Indiana University Press.
171 Carrillo, J, G Koumoundouros, P Divanach, and J Martinez. 2001. Morphological malformations of the lateral line in reared gilthead sea bream (Sparus aurata L. 1758). Aquaculture 192: 281–290. https://doi.org/10.1016/S0044-8486(00)00454-3.
172 Bleckmann, Horst. 1986. Role of the Lateral Line in Fish Behaviour. In The Behaviour of Teleost Fishes, ed. Tony J. Pitcher, 177–202. Springer US.
173 Imre, I., J. W. A. Grant, and R. A. Cunjak. 2005. Density-dependent growth of young-of-the-year Atlantic salmon Salmo salar in Catamaran Brook, New Brunswick. Journal of Animal Ecology 74: 508–516. https://doi.org/10.1111/j.1365-2656.2005.00949.x.
174 Réale, Denis, Simon M. Reader, Daniel Sol, Peter T. McDougall, and Niels J. Dingemanse. 2007. Integrating animal temperament within ecology and evolution. Biological Reviews 82: 291–318. https://doi.org/10.1111/j.1469-185X.2007.00010.x.
175 Robb, D H F, and S C Kestin. 2002. Methods Used to Kill Fish: Field Observations and Literature Reviewed. Animal Welfare 11: 269–282.
176 Robb, D. H. F., S. B. Wotton, J. L. McKinstry, N. K. Sørensen, S. C. Kestin, and N. K. Sørensen. 2000. Commercial slaughter methods used on Atlantic salmon: determination of the onset of brain failure by electroencephalography. Veterinary Record 147: 298–303. https://doi.org/10.1136/vr.147.11.298.
❮
❯







«