Information
Version: B | 1.1 (2022-06-23)
WelfareScore | farm
Condensed assessment of the species' likelihood and potential for good fish welfare in aquaculture, based on ethological findings for 10 crucial criteria.
- Li = Likelihood that the individuals of the species experience good welfare under minimal farming conditions
- Po = Potential of the individuals of the species to experience good welfare under high-standard farming conditions
- Ce = Certainty of our findings in Likelihood and Potential
WelfareScore = Sum of criteria scoring "High" (max. 10)
General remarks
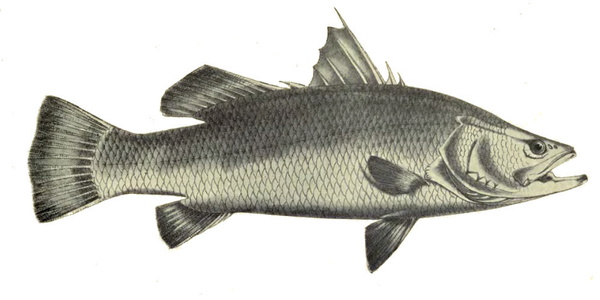
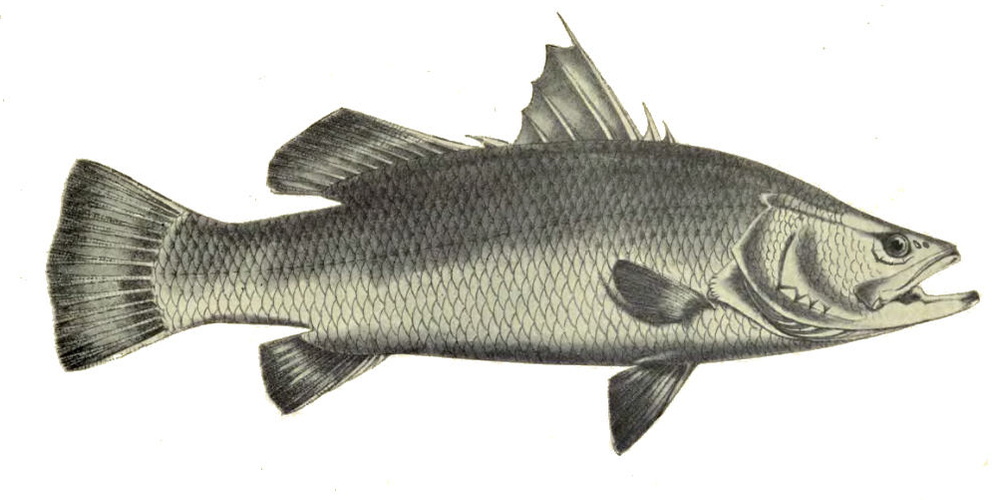
Lates calcarifer is an important coastal, estuarine, and freshwater fish in the Indo-Pacific region. Aquaculture of this species began in the 1970s in Thailand and rapidly spread throughout much of Southeast Asia. Its delicately flavoured meat, fast growth rate, large size, and easy breeding in captivity make it a very attractive species for aquaculture. However, in general, the current available rearing techniques need to be optimised for improving fish welfare as demonstrated in almost all the criteria below. Some limitations of this species have already been identified, such as cannibalism in early life stages that may be improved by using rearing systems with low light intensity and refuges. Further research is needed to identify possible long-term effects on welfare. Stress by pre-slaughter and slaughter method can be avoided using a rested harvest technique. Future research and developmental work should therefore be directed towards resolving some of these welfare limitations.
1 Home range
Many species traverse in a limited horizontal space (even if just for a certain period of time per year); the home range may be described as a species' understanding of its environment (i.e., its cognitive map) for the most important resources it needs access to.
What is the probability of providing the species' whole home range in captivity?
It is unclear for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


2 Depth range
Given the availability of resources (food, shelter) or the need to avoid predators, species spend their time within a certain depth range.
What is the probability of providing the species' whole depth range in captivity?
It is low for minimal and high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


3 Migration
Some species undergo seasonal changes of environments for different purposes (feeding, spawning, etc.), and to move there, they migrate for more or less extensive distances.
What is the probability of providing farming conditions that are compatible with the migrating or habitat-changing behaviour of the species?
It is low for minimal and high-standard farming conditions. Our conclusion is based on a high amount of evidence.


4 Reproduction
A species reproduces at a certain age, season, and sex ratio and possibly involving courtship rituals.
What is the probability of the species reproducing naturally in captivity without manipulation of theses circumstances?
It is low for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


5 Aggregation
Species differ in the way they co-exist with conspecifics or other species from being solitary to aggregating unstructured, casually roaming in shoals or closely coordinating in schools of varying densities.
What is the probability of providing farming conditions that are compatible with the aggregation behaviour of the species?
It is unclear for minimal and high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


6 Aggression
There is a range of adverse reactions in species, spanning from being relatively indifferent towards others to defending valuable resources (e.g., food, territory, mates) to actively attacking opponents.
What is the probability of the species being non-aggressive and non-territorial in captivity?
It is low for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


7 Substrate
Depending on where in the water column the species lives, it differs in interacting with or relying on various substrates for feeding or covering purposes (e.g., plants, rocks and stones, sand and mud, turbidity).
What is the probability of providing the species' substrate and shelter needs in captivity?
It is low for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


8 Stress
Farming involves subjecting the species to diverse procedures (e.g., handling, air exposure, short-term confinement, short-term crowding, transport), sudden parameter changes or repeated disturbances (e.g., husbandry, size-grading).
What is the probability of the species not being stressed?
It is low for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a high amount of evidence.


9 Malformations
Deformities that – in contrast to diseases – are commonly irreversible may indicate sub-optimal rearing conditions (e.g., mechanical stress during hatching and rearing, environmental factors unless mentioned in crit. 3, aquatic pollutants, nutritional deficiencies) or a general incompatibility of the species with being farmed.
What is the probability of the species being malformed rarely?
It is low for minimal and high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


10 Slaughter
The cornerstone for a humane treatment is that slaughter a) immediately follows stunning (i.e., while the individual is unconscious), b) happens according to a clear and reproducible set of instructions verified under farming conditions, and c) avoids pain, suffering, and distress.
What is the probability of the species being slaughtered according to a humane slaughter protocol?
It is low for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


Side note: Domestication
Teletchea and Fontaine introduced 5 domestication levels illustrating how far species are from having their life cycle closed in captivity without wild input, how long they have been reared in captivity, and whether breeding programmes are in place.
What is the species’ domestication level?
DOMESTICATION LEVEL 4 34, level 5 being fully domesticated.
Side note: Forage fish in the feed
450-1,000 milliard wild-caught fishes end up being processed into fish meal and fish oil each year which contributes to overfishing and represents enormous suffering. There is a broad range of feeding types within species reared in captivity.
To what degree may fish meal and fish oil based on forage fish be replaced by non-forage fishery components (e.g., poultry blood meal) or sustainable sources (e.g., soybean cake)?
All age classes: WILD: carnivorous 35. FARM: fish meal and fish oil may be partly* replaced by non-forage fishery components 36 37 38 39 40.
*partly = <51% – mostly = 51-99% – completely = 100%
Glossary
CATADROMOUS = migrating from fresh water into the sea to spawn
DEMERSAL = living and feeding on or near the bottom of a body of water, mostly benthopelagic, some benthic
DOMESTICATION LEVEL 4 = entire life cycle closed in captivity without wild inputs 34
FARM = setting in farming environment or under conditions simulating farming environment in terms of size of facility or number of individuals
IND = individuals
JUVENILES = fully developed but immature individuals, for details ➝ Findings 10.1 Ontogenetic development
LAB = setting in laboratory environment
LARVAE = hatching to mouth opening, for details ➝ Findings 10.1 Ontogenetic development
PHOTOPERIOD = duration of daylight
SPAWNERS = adults during the spawning season; in farms: adults that are kept as broodstock
WILD = setting in the wild
Bibliography
2 Kungvankij, P., and N. Suthemechaikul. 1986. Mass Production of Seabass, Lates calcarifer (Bloch) by Environmental Manipulation. NACA/WP/86/48. Network of aquaculture centres in Asia Bangkok, Thailand.
3 Russell, D. J., and R. N. Garrett. 1985. Early life history of barramundi, Lates calcarifer (Bloch), in north-eastern Queensland. Marine and Freshwater Research 36: 191–201. https://doi.org/10.1071/mf9850191.
4 Blaber, Stephen J. M., David A. Milton, and John P. Salini. 2008. Chapter 11 The Biology of Barramundi (Lates calcarifer) in the Fly River System. In Developments in Earth and Environmental Sciences, ed. Barrie Bolton, 9:411–426. The Fly River, Papu a New Guinea: Environmental Studies in an Impacted Tropical River System. Elsevier. https://doi.org/10.1016/S1571-9197(08)00411-4.
5 Moore, R. 1982. Spawning and early life history of Barramundi, Lates calcarifer (Bloch), in Papua New Guinea. Australian journal of marine and freshwater research 33: 647–61.
6 Parazo, Monina M., Luis Ma B. Garcia, Felix G. Ayson, Armando C. Fermin, Jesus M. E. Almendras, Reyes Jr, Deogracias M, Enrique M. Avila, and Joebert D. Toledo. 1998. Sea bass hatchery operations. Aquaculture Department, Southeast Asian Fisheries Development Center.
7 Parazo, M. M., E. M. Avila, and D. M. Reyes Jr. 1991. Size-and weight-dependent cannibalism in hatchery-bred sea bass (Lates calcarifer Bloch). Journal of Applied Ichthyology 7: 1–7. https://doi.org/10.1111/j.1439-0426.1991.tb00588.x.
8 Mathew, Grace. 2009. Taxonomy, identification and biology of Seabass (Lates calcarifer). In Course manual: National training on cage culture of seabass, ed. Joseph Imelda, V. Edwin Joseph, and V. Susmitha, 38–43. Kochi: CMFRI & NFDB.
9 Jerry, D. R. 2013. Biology and Culture of Asian Seabass Lates Calcarifer.
10 Krishnamurthy, K., and M.J. Prince Jeyaseelan. 1981. The early life history of fishes from Pichavaram mangrove ecosystem of India. Réun. Cons. int. Explor. Mer 178: 416–423.
11 Pender, Peter J., and Roland K. Griffin. 1996. Habitat History of Barramundi Lates calcarifer in a North Australian River System Based on Barium and Strontium Levels in Scales. Transactions of the American Fisheries Society 125: 679–689. https://doi.org/10.1577/1548-8659(1996)125<0679:HHOBCI>2.3.CO;2.
12 Riede, K. 2004. Global register of migratory species - from global to regional scales. Final report of the R&D Projekt 808 05 081. Bonn, Germany: Federal Agency for Nature Conservation.
13 Reynolds, L. F., and R. Moore. 1982. Growth rates of barramundi, Lates calcarifer (Bloch), in Papua new Guinea. Marine and Freshwater Research 33: 663–670. https://doi.org/10.1071/mf9820663.
14 Kungvankij, P., L.B. Tiro, B.J. Pudadera, and I.O. Potesta. 1985. Training Manual Biology and Culture of Sea Bass (Lates calcarifer). NACA/TR/85/13. Bangkok, Thailand: Network of Aquaculture Centres in Asia.
15 Davis, T. L. O. 1986. Migration patterns in barramundi, Lates calcarifer (Bloch), in Van Diemen Gulf, Australia, with estimates of fishing mortality in specific areas. Fisheries Research 4: 243–258. https://doi.org/10.1016/0165-7836(86)90006-8.
16 Jesus-Ayson, De, Evelyn Grace, Felix G. Ayson, and Valentin Thepot. 2014. Early development and seed production of Asian seabass, Lates calcarifer. In Biology and Culture of Asian Seabass Lates Calcarifer, 17–30. CRC Press. https://doi.org/10.1201/b15974-3.
17 Garrett, RN, and JJ O’Brien. 1994. All year-round spawning of hatchery barramundi in Australia 8: 40–42.
18 Sadhu, Narasimhulu, S. R. Krupesha Sharma, Shoji Joseph, Praveen Dube, and K. K. Philipose. 2014. Chronic stress due to high stocking density in open sea cage farming induces variation in biochemical and immunological functions in Asian seabass (Lates calcarifer, Bloch). Fish Physiology and Biochemistry 40: 1105–1113. https://doi.org/10.1007/s10695-014-9909-8.
19 Ardiansyah, and Ravi Fotedar. 2016. Water quality, growth and stress responses of juvenile barramundi (Lates calcarifer Bloch), reared at four different densities in integrated recirculating aquaculture systems. Aquaculture 458: 113–120. https://doi.org/10.1016/j.aquaculture.2016.03.001.
20 Kailola, P., Meryl Williams, P. Stewart, R. Reichelt, A. McNee, and Chris Grieve. 1993. Australian Fisheries Resources.
21 Kailasam, Muniyandi, A.R. Thirunavukkarasu, M Abraham, P Kishore Chandra, and Subburaj Ramasubbu. 2002. Influence of size variation and feeding on cannibalism of Asian seabass Lates calcarifer (Bloch) during hatchery rearing phase. Indian J. Fish. 49: 107–113.
22 Ribeiro, Flavio F., and Jian G. Qin. 2013. Modelling Size-Dependent Cannibalism in Barramundi Lates calcarifer: Cannibalistic Polyphenism and Its Implication to Aquaculture. PLOS ONE 8: e82488. https://doi.org/10.1371/journal.pone.0082488.
23 Rabanal, H.S., and V. Soesanto. 1982. Report of the training course on seabass spawning and larval rearing. Songkhla, Thailand: Food and Agriculture Organization of the United Nations.
24 Appelbaum, S., and A. Jesus Arockiaraj. 2010. Sibling cannibalism in juvenile Asian sea bass ( Lates calcarifer ) reared under different photoperiods. AACL Bioflux 3: 384–392.
25 Qin, J.G., L. Mittiga, and F. Ottolenghi. 2004. Cannibalism Reduction in Juvenile Barramundi Lates calcarifer by Providing Refuges and Low Light. Journal of the World Aquaculture Society 35.
26 Barlow, Christopher G. 1998. Aspects of the biology of juvenile barramundi Lates calcarifer (Bloch) relevant to production for recreational fisheries and farming, with a note on the proposal to introduce Nile perch Lates niloticus (L.) to Australia. Phd, James Cook University.
27 Dhert, Philippe, Patrick Lavens, Marietta Duray, and Patrick Sorgeloos. 1990. Improved larval survival at metamorphosis of Asian seabass (Lates calcarifer) using ω3-HUFA-enriched live food. Aquaculture 90: 63–74. https://doi.org/10.1016/0044-8486(90)90283-S.
28 Lestang, P., Roland K. Griffin, and Q.A. Allsop. 2004. Physiology of barramundi (Lates calcarifer). 73. Northern Territory Government.
29 Newton, J. R., C. De Santis, and D. R. Jerry. 2012. The gene expression response of the catadromous perciform barramundi Lates calcarifer to an acute heat stress. Journal of Fish Biology 81: 81–93. https://doi.org/10.1111/j.1095-8649.2012.03310.x.
30 Wilkinson, Ryan J., Nicholas Paton, and Mark J. R. Porter. 2008. The effects of pre-harvest stress and harvest method on the stress response, rigor onset, muscle pH and drip loss in barramundi (Lates calcarifer). Aquaculture 282: 26–32. https://doi.org/10.1016/j.aquaculture.2008.05.032.
31 Fraser, M. R., T. A. Anderson, and R. de Nys. 2004. Ontogenic development of the spine and spinal deformities in larval barramundi (Lates calcarifer) culture. Aquaculture 242: 697–711. https://doi.org/10.1016/j.aquaculture.2004.09.018.
32 Fraser, M. R., and R. de Nys. 2005. The morphology and occurrence of jaw and operculum deformities in cultured barramundi (Lates calcarifer) larvae. Aquaculture 250: 496–503. https://doi.org/10.1016/j.aquaculture.2005.04.067.
33 Cobcroft, Jennifer M., and Stephen C. Battaglene. 2013. Skeletal malformations in Australian marine finfish hatcheries. Aquaculture 396–399: 51–58. https://doi.org/10.1016/j.aquaculture.2013.02.027.
34 Teletchea, Fabrice, and Pascal Fontaine. 2012. Levels of domestication in fish: implications for the sustainable future of aquaculture. Fish and Fisheries 15: 181–195. https://doi.org/10.1111/faf.12006.
35 Tait, R. D. 1981. Comparison of the diets of the northern spotted barramundi (Scleropages jardini) and the giant perch (Lates calcarifer) in northern Australia. Proceedings - International Association of Theoretical and Applied Limnology.
36 Boonyaratpalin, M, P Suraneiranat, and T Tunpibal. 1998. Replacement of fish meal with various types of soybean products in diets for the Asian seabass, Lates calcarifer. Aquaculture 161: 67–78. https://doi.org/10.1016/S0044-8486(97)00257-3.
37 Raso, Sayam, and Trevor A Anderson. 2003. Effects of dietary fish oil replacement on growth and carcass proximate composition of juvenile barramundi (Lates calcarifer). Aquaculture Research 34: 813–819. https://doi.org/10.1046/j.1365-2109.2003.00885.x.
38 Glencross, B., N. Rutherford, and B. Jones. 2011. Evaluating options for fishmeal replacement in diets for juvenile barramundi (Lates calcarifer). Aquaculture Nutrition 17: e722–e732. https://doi.org/10.1111/j.1365-2095.2010.00834.x.
39 Glencross, Brett, David Blyth, Simon Irvin, Nicholas Bourne, Marcel Campet, Pascal Boisot, and Nicholas M. Wade. 2016. An evaluation of the complete replacement of both fishmeal and fish oil in diets for juvenile Asian seabass, Lates calcarifer. Aquaculture 451: 298–309. https://doi.org/10.1016/j.aquaculture.2015.09.012.
40 Katya, Kumar, M. Z. S. Borsra, Dev Ganesan, Giva Kuppusamy, Max Herriman, Andrew Salter, and Sayed Azam Ali. 2017. Efficacy of insect larval meal to replace fish meal in juvenile barramundi, Lates calcarifer reared in freshwater. International Aquatic Research 9: 303–312. https://doi.org/10.1007/s40071-017-0178-x.