Information
Version: B | 1.1 (2022-01-22)
1 Remarks
1.1 General remarks
Escapees and consequences: negative or at most unpredictable for the local ecosystem1.2 Other remarks
No data found yet.2 Ethograms
In the farm or lab: on daily rhythm, reproduction, communication, social behaviour, cognitive abilities, stress reactions
3 Distribution


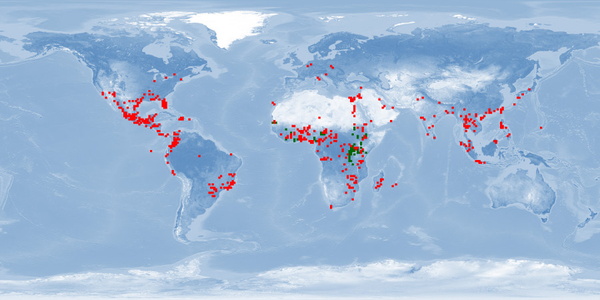
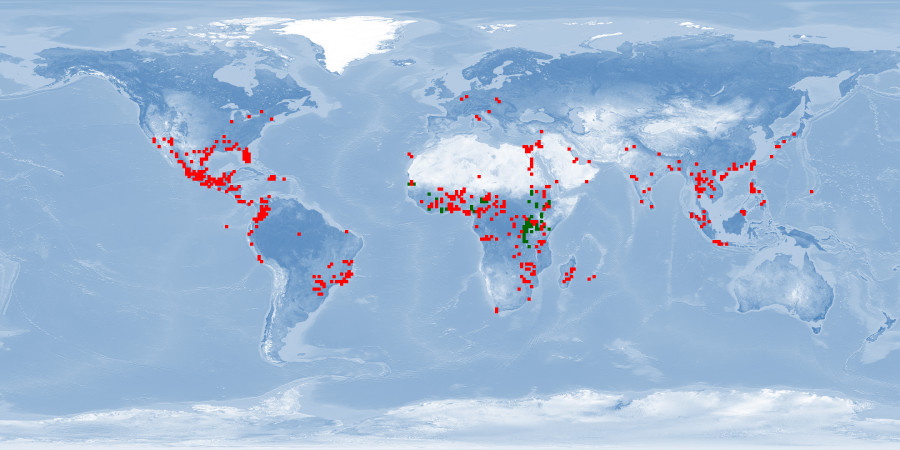
Natural distribution: Africa
Introduced: Africa, North America
4 Natural co-existence
5 Substrate and/or shelter
5.1 Substrate
Substrate range, substrate preference: lives over sand and mudSubstrate and stress: enrichment increases number of fights (further research needed)
Substrate and growth: direct effect (further research needed)
5.2 Shelter or cover
Shelter or cover preference: complex structures or artificial alternatives (further research needed)6 Food, foraging, hunting, feeding
6.1 Trophic level and general considerations on food needs
Trophic level: 2.0Impacts of feed fishery: contributes to overfishing, challenges animal welfare
6.2 Food items
Food items, food preference: opportunistic – either mainly herbivorous or mainly omnivorous6.3 Feeding behaviour
Feeding style, foraging mode: depending on diet either bottom grazing or active pursuitFeeding frequency and growth: direct relation (further research needed)
Food competition and stress: direct effect (further research needed)
For feeding and exploration-avoidance continuum ➝ D3.
7 Photoperiod
7.1 Daily rhythm
Daily rhythm: large inter-individual differences (further research needed)Photoperiod and growth: direct relation (further research needed)
7.2 Light intensity
Light intensity and stress: direct relation (further research needed)7.3 Light colour
Light colour and stress: no effect of white, blue, or yellow light (further research needed)Light colour and growth: lowest variation under yellow light (further research needed)
For light colour and...
...nest building ➝ D4,
...mouthbreeding ➝ D5,
...confinement ➝ D6.
8 Water parameters
8.1 Water temperature
Standard temperature range, temperature preference: 16-29 °CTemperature and stress: decreasing survival <25 °C and >30 °C, but depends on acclimatisation (further research needed)
Temperature and growth: optimally 30 °C (further research needed)
8.2 Oxygen
Dissolved oxygen range: 0-12 mg/L8.3 Salinity
Salinity tolerance, standard salinity range: probably euryhaline (further research needed)Salinity and stress: survives 35‰ if gradually exposed (further research needed)
8.4 pH
Standard pH range: 7.9-8.0 (further research needed)8.5 Turbidity
Standard turbidity range: Secchi depth 0.2-0.8 m8.6 Water hardness
No data found yet.8.7 NO4
No data found yet.8.8 Other
No data found yet.9 Swimming
9.1 Swimming type, swimming mode
Swimming type, swimming mode: carangiform9.2 Swimming speed
No data found yet.9.3 Home range
No data found yet.9.4 Depth
Depth range, depth preference: 2-6+ m, up to 20 m (further research needed)9.5 Migration
No data found yet.10 Growth
10.1 Ontogenetic development
Mature egg: 4-5 days from fertilisation until hatching, 1.5-2.5 mm diameter (further research needed)Larvae: hatching to 6-12 days, 4-5+ mm, 1.7-3.7 mg
Fry: beginning of exogenous feeding (at 4-8 days),10+ mm
Juveniles, sexual maturity: fully developed to beginning of maturity (at 2-7 months), 7-37 cm, 1.8-90 g
Adults: 2+ months, 7.3-56 cm, 17.2-4,300 g
10.2 Sexual conversion
Sex and manipulation: elevated water temperature, androgen treatment, breeding programme increases portion of male fry10.3 Sex ratio
Natural male:female ratio: 1:1-2:110.4 Effects on growth
Growth and sex: bimodal pattern, noticeable from 2-5 months onGrowth and size-grading: no effect (further research needed)
Growth and other factors: direct effect of female's age, direct effect of polyculture with Clarias gariepinus (further research needed)
For growth and...
...substrate ➝ D11,
...particle size ➝ D2,
...feeding frequency ➝ D12,
...PHOTOPERIOD ➝ D13,
...light colour ➝ D14,
...water temperature ➝ D15,
...stocking density ➝ D16.
10.5 Deformities and malformations
No data found yet.11 Reproduction
11.1 Nest building
Nest building: males and females build nests in sand (further research needed)Effects on nest building: blue light, olfaction, dominance benefits nest building (further research needed)
11.2 Attraction, courtship, mating
Attraction: reddish-brown colour, swollen urogenital papillaCourtship sequence: male nudges female, leads her to spawning site (further research needed)
11.3 Spawning
r or K selection: flexibleMating system: flexible
Spawning conditions: no substrate necessary (further research needed), several yearly spawnings, >20 °C
Male:female ratio resulting in spawning, composition of the broodstock: 1:1-1:10
Spawning sequence: 45-120 min (further research needed)
Effects on spawning: only dominant males spawn, visual contact necessary (further research needed)
11.4 Fecundity
Female fecundity: 20-6,000+ eggs per batch in several batchesMale fecundity: 2-4 times a day
Effects on fecundity: concrete tanks superior to hapa nets (further research needed)
11.5 Brood care, breeding
Breeding type: mouthbreeder: female takes eggs into mouth directly after fertilisation and carries 39-241 eggs in her buccal cavity for 7-18 days through hatching and conversion into fry (further research needed)Effects on breeding: blue light, round-bottom hatching jars benefit breeding (further research needed)
12 Senses
12.1 Vision
Visible spectrum: violet, blue, green, yellow, red (further research needed)12.2 Olfaction (and taste, if present)
Importance of olfaction: risk perception, nest building (further research needed)12.3 Hearing
Hearing type, hearing spectrum: hearing generalist, 100-800 Hz (further research needed)Noise and stress: hearing loss from long-term noise (further research needed)
12.4 Touch, mechanical sensing
No data found yet.12.5 Lateral line
No data found yet.12.6 Electrical sensing
No data found yet.12.7 Nociception, pain sensing
Nociception spectrum: anomalous behaviour and decrease of mucus cells after tail clip (further research needed)12.8 Other
No data found yet.13 Communication
13.1 Visual
For visual communication......and courtship ➝ D17,
...spawning ➝ D18.
13.2 Chemical
No data found yet.13.3 Acoustic
Sounds during nest defence: pulse trains of 2-4 pulses of 114 ms each and 57 HzSounds during feeding: sounds of 2,870 Hz for 23 ms
13.4 Mechanical
No data found yet.13.5 Electrical
No data found yet.13.6 Other
No data found yet.14 Social behaviour
14.1 Spatial organisation
Aggregation type: shoal (further research needed)Stocking density and stress: direct relation from ca 50 ind/m3 on (further research needed)
Stocking density and growth: inverse relation from ca 50 ind/m3 on (further research needed)
14.2 Social organisation
Social organisation type: usually linear hierarchy (when in small groups)Features of dominance: lighter body and eye colour, more aggressive than subordinate
Features of subordination: darker body and eye colour than dominant, flee attacks, avoid contact with the dominant
14.3 Exploitation
No data found yet.14.4 Facilitation
No data found yet.14.5 Aggression
Aggression and size-grading: biting, chasing, fights regardless of size-grading via linear hierarchy (further research needed)Effects on aggression: no sex bias in juveniles (further research needed)
For aggression and...
...environmental enrichment ➝ D21,
...light intensity ➝ D22,
...predator recognition ➝ D23.
14.6 Territoriality
For territoriality and......sounds during nest defence ➝ D24,
...hierarchy ➝ D25,
...predator recognition ➝ D23.
15 Cognitive abilities
15.1 Learning
Classical conditioning: failed with food as unconditioned stimulus (further research needed)Operant or instrumental conditioning: may be used for managing self-feeder, initiating flight response
15.2 Memory
No data found yet.15.3 Problem solving, creativity, planning, intelligence
No data found yet.15.4 Other
Predator recognition: innate16 Personality, coping styles
Exploration-avoidance continuum: relationship with feeding resumption, ventilatory frequency, boldness (further research needed)
Aggressiveness continuum: in establishing hierarachy, dominance-subordination, given size-grading
17 Emotion-like states
17.1 Joy
No data found yet.17.2 Relaxation
No data found yet.17.3 Sadness
No data found yet.17.4 Fear
Fear: associated with confinement situation (further research needed)18 Self-concept, self-recognition
19 Reactions to husbandry
19.1 Stereotypical and vacuum activities
No data found yet.19.2 Acute stress
Handling: chasing for 60 s, netting is stressful (further research needed)Confinement: already pretending to confine is stressful (further research needed)
For acute stress and...
...food competition ➝ D31,
...light intensity ➝ D22,
...light colour ➝ D32,
...olfaction ➝ D33,
...pain ➝ D30,
...linear hierarchy in small groups ➝ D27,
...coping styles ➝ D34 D3,
...fear ➝ D35,
...stunning ➝ D36.
19.3 Chronic stress
For chronic stress and......environmental enrichment ➝ D21,
...water temperature ➝ D37,
...salinity ➝ D38,
...rearing container ➝ D5,
...noise ➝ D39,
...stocking density ➝ D40,
...hierarchy ➝ D27,
...personality ➝ D3.
19.4 Stunning reactions
Stunning rules: fast, effective, safeStunning methods: electrical stunning most effective (further research needed)
Stunning methods and stress: asphyxiation in 50:50 ice water mixture more stressful (further research needed)
Glossary
AGGRESSIVENESS = agonistic reactions towards conspecifics. Tests: mirror image, social interaction/diadic encounters 100.
EXPLORATION-AVOIDANCE = reaction to new situations, e.g. new habitat, new food, novel objects. Referred to as neophobia/neophilia elsewhere. Tests: open field, trappability for first time, novel environment, hole board (time spent with head in holes), novel object 100.
FARM = setting in farming environment or under conditions simulating farming environment in terms of size of facility or number of individuals
FINGERLINGS = early juveniles with fully developed scales and working fins, the size of a human finger; for details ➝ Findings 10.1 Ontogentic development
FOOD CONVERSION RATIO = (food offered / weight gained)
FRY = larvae from external feeding on, for details ➝ Findings 10.1 Ontogenetic development
GENERALIST = Generalists detect a narrow bandwidth of sound frequencies (<50-500 Hz, 1,500 Hz max.). High hearing threshold = cannot detect quieter sounds. Typically no swim bladder or no attachment of the swim bladder to the inner ear. Live in loud environments (rivers) 97 98.
IND = individuals
JUVENILES = fully developed but immature individuals, for details ➝ Findings 10.1 Ontogenetic development
LAB = setting in laboratory environment
LARVAE = hatching to mouth opening, for details ➝ Findings 10.1 Ontogenetic development
MILLIARD = 1,000,000,000 51 52
PHOTOPERIOD = duration of daylight
POST-LARVAE = fully developed individuals, beginning of external sex differentiation; for details ➝ Findings 10.1 Ontogenetic development
SHYNESS-BOLDNESS = reaction to risky (but not new!) situations, e.g. predators or humans. Referred to as docility, tameness, fearfulness elsewhere. Tests: predator presentation, predator stimulus, threat, trappability (latency to enter a trap for first time can be exploration), resistance to handlers (Trapezov stick test), tonic immobility (catatonic-like death-feigning anti predator response) 100.
TOTAL LENGTH = from snout to tip of caudal fin as compared to fork length (which measures from snout to fork of caudal fin) 74 or standard length (from head to base of tail fin) or body length (from the base of the eye notch to the posterior end of the telson)
WILD = setting in the wild
Bibliography
2 Peterson, Mark S., William T. Slack, Nancy J. Brown-Peterson, Jennifer L. McDonald, and C. M. Taylor. 2004. Reproduction in Nonnative Environments: Establishment of Nile Tilapia, Oreochromis niloticus, in Coastal Mississippi Watersheds. Copeia 2004: 842–849. https://doi.org/10.1643/CE-04-134R1.
3 Komolafe, O. O., and G. a. O. Arawomo. 2007. Reproductive strategy of Oreochromis niloticus (Pisces: Cichlidae) in Opa reservoir, Ile-Ife, Nigeria. Revista de Biología Tropical 55: 595–602.
4 Toguyeni, Aboubacar, Benoit Fauconneau, Thierry Boujard, Alexis Fostier, Eduard R Kuhn, Koen A Mol, and Jean-Francois Baroiller. 1997. Feeding Behaviour and Food Utilisation in Tilapia, Oreochromis Niloticus: Effect of Sex Ratio and Relationship With the Endocrine Status. Physiology & Behavior 62: 273–279. https://doi.org/10.1016/S0031-9384(97)00114-5.
5 Vera, Luisa María, Louise Cairns, Francisco Javier Sánchez-Vázquez, and Hervé Migaud. 2009. Circadian Rhythms of Locomotor Activity in the Nile Tilapia Oreochromis niloticus. Chronobiology International 26: 666–681. https://doi.org/10.1080/07420520902926017.
6 Volpato, G. L., C. R. A. Duarte, and A. C. Luchiari. 2004. Environmental color affects Nile tilapia reproduction. Brazilian Journal of Medical and Biological Research 37: 479–483. https://doi.org/10.1590/S0100-879X2004000400004.
7 Uchida, Hiroshi, Satoshi Ogawa, Mina Harada, Masato Matushita, Munehico Iwata, Yasuo Sakuma, and Ishwar S. Parhar. 2005. The olfactory organ modulates gonadotropin-releasing hormone types and nest-building behavior in the tilapia Oreochromis niloticus. Journal of Neurobiology 65: 1–11. https://doi.org/10.1002/neu.20156.
8 Mendonça, Francine Z., and Eliane Gonçalves-de-Freitas. 2008. Nest deprivation and mating success in Nile tilapia (Teleostei: Cichlidae). Revista Brasileira de Zoologia 25: 413–418. https://doi.org/10.1590/S0101-81752008000300005.
9 Castro, A. L. S., E. Gonçalves-de-Freitas, G. L. Volpato, and C. Oliveira. 2009. Visual communication stimulates reproduction in Nile tilapia, Oreochromis niloticus (L.). Brazilian Journal of Medical and Biological Research 42: 368–374. https://doi.org/10.1590/S0100-879X2009000400009.
10 Mendonça, F. Z., G. L. Volpato, R. S. Costa-Ferreira, and E. Gonçalves-de-Freitas. 2010. Substratum choice for nesting in male Nile tilapia Oreochromis niloticus. Journal of Fish Biology 77: 1439–1445. https://doi.org/10.1111/j.1095-8649.2010.02754.x.
11 Rana, Kausik J. 1986. Parental influences on egg quality, fry production and fry performance in Oreochromis niloticus (Linnaeus) and O. mossambicus (Peters). Doctoral dissertation, U.K.: University of Stirling.
12 Longrie, Nicolas, Sam Van Wassenbergh, Pierre Vandewalle, Quentin Mauguit, and Eric Parmentier. 2009. Potential mechanism of sound production in Oreochromis niloticus (Cichlidae). Journal of Experimental Biology 212: 3395–3402. https://doi.org/10.1242/jeb.032946.
13 Delcourt, Johann, Christophe Becco, Nicolas Vandewalle, and Pascal Poncin. 2009. A video multitracking system for quantification of individual behavior in a large fish shoal: Advantages and limits. Behavior Research Methods 41: 228–235. https://doi.org/10.3758/BRM.41.1.228.
14 Barreto, Rodrigo Egydio, Ana Carolina Luchiari, and Ana Lucia Marcondes. 2003. Ventilatory frequency indicates visual recognition of an allopatric predator in naı̈ve Nile tilapia. Behavioural Processes 60: 235–239. https://doi.org/10.1016/S0376-6357(02)00127-4.
15 Volpato, G. L., A. C. Luchiari, C. R. A. Duarte, R. E. Barreto, and G. C. Ramanzini. 2003. Eye color as an indicator of social rank in the fish Nile tilapia. Brazilian Journal of Medical and Biological Research 36: 1659–1663. https://doi.org/10.1590/S0100-879X2003001200007.
16 Corrêa, S. A., M. O. Fernandes, K. K. Iseki, and J. A. Negrão. 2003. Effect of the establishment of dominance relationships on cortisol and other metabolic parameters in Nile tilapia (Oreochromis niloticus). Brazilian Journal of Medical and Biological Research 36: 1725–1731. https://doi.org/10.1590/S0100-879X2003001200015.
17 Gonçalves-de-Freitas, Eliane, and Aline Chimello Ferreira. 2004. Female social dominance does not establish mating priority in Nile tilapia. Revista de Etologia 6: 33–37.
18 Barbosa, J. M., S. S. S. Brugiolo, J. Carolsfeld, and S. S. Leitão. 2006. Heterogeneous growth fingerlings of the Nile tilapia Oreochromis niloticus: effects of density and initial size variability. Brazilian Journal of Biology 66: 537–541. https://doi.org/10.1590/S1519-69842006000300020.
19 Barreto, Rodrigo Egydio, and Gilson Luiz Volpato. 2006. Stress responses of the fish Nile tilapia subjected to electroshock and social stressors. Brazilian Journal of Medical and Biological Research 39: 1605–1612. https://doi.org/10.1590/S0100-879X2006001200012.
20 Barreto, Rodrigo Egydio, and Gilson Luiz Volpato. 2006. Ventilatory frequency of Nile tilapia subjected to different stressors. Journal of Experimental Animal Science 43: 189–196. https://doi.org/10.1016/j.jeas.2006.05.001.
21 Evans, Joyce J., David J. Pasnik, Patrick Horley, Kimberly Kraeer, and Phillip H. Klesius. 2008. Aggression and Mortality among Nile Tilapia (Oreochromis niloticus) Maintained in the Laboratory at Different Densities. Research Journal of Animal Sciences 2: 57–64.
22 Carvalho, Thaís B., Francine Z. Mendonça, Roselene S. Costa-Ferreira, and Eliane Gonçalves-de-Freitas. 2013. The effect of increased light intensity on the aggressive behavior of the Nile tilapia, Oreochromis niloticus (Teleostei: Cichlidae). Zoologia (Curitiba) 30: 125–129. https://doi.org/10.1590/S1984-46702013000200001.
23 Pinho-Neto, Candido Ferreira, Caio Akira Miyai, Fabio Henrique Carretero Sanches, Percilia Cardoso Giaquinto, Helton Carlos Delicio, Leonardo José Gil Barcellos, Gilson Luiz Volpato, and Rodrigo Egydio Barreto. 2014. Does sex influence intraspecific aggression and dominance in Nile tilapia juveniles? Behavioural Processes 105: 15–18. https://doi.org/10.1016/j.beproc.2014.02.003.
24 Barreto, Rodrigo Egydio, Graziele G. Arantes Carvalho, and Gilson Luiz Volpato. 2011. The aggressive behavior of Nile tilapia introduced into novel environments with variation in enrichment. Zoology 114: 53–57. https://doi.org/10.1016/j.zool.2010.09.001.
25 Barreto, Rodrigo Egydio, and Gilson Luiz Volpato. 2007. Evaluating feeding as unconditioned stimulus for conditioning of an endocrine effect in Nile tilapia. Physiology & Behavior 92: 867–872. https://doi.org/10.1016/j.physbeh.2007.06.013.
26 Martins, Catarina I. M., Patricia I. M. Silva, Luis E. C. Conceição, Benjamin Costas, Erik Höglund, Øyvind Øverli, and Johan W. Schrama. 2011. Linking fearfulness and coping styles in fish. PLOS ONE 6: e28084. https://doi.org/10.1371/journal.pone.0028084.
27 Barreto, Rodrigo Egydio, and Gilson Luiz Volpato. 2011. Ventilation rates indicate stress-coping styles in Nile tilapia. Journal of Biosciences 36: 851–855. https://doi.org/10.1007/s12038-011-9111-4.
28 Barreto, Rodrigo Egydio, and Gilson Luiz Volpato. 2004. Caution for using ventilatory frequency as an indicator of stress in fish. Behavioural Processes 66: 43–51. https://doi.org/10.1016/j.beproc.2004.01.001.
29 Maia, Caroline Marques, and Gilson Luiz Volpato. 2013. Environmental light color affects the stress response of Nile tilapia. Zoology 116: 64–66. https://doi.org/10.1016/j.zool.2012.08.001.
30 Reviewed distribution maps for Nile tilapia (Oreochromis niloticus). 2016. Aquamaps.
31 Trewavas, Ethelwynn, and Guy G. Teugels. 1991. Sarotherodon. In Check-list of the Freshwater Fishes of Africa: Cloffa, ed. Jacques Daget, J. P. Gosse, Guy G. Teugels, and Dirk F. E. Thys van den Audenaerde, 4:425–437. Brussels: ISNB.
32 Froese, R., and D. Pauly. 2014. FishBase. World Wide Web electronic publication. www.fishbase.org.
33 Trewavas, Ethelwynn. 1983. Tilapiine Fishes of the Genera Sarotherodon, Oreochromis and Danakilia. [Published in cooperation with] British Museum (Natural History) [by] Comstock Pub. Associates, a division of Cornell University Press.
34 Teugels, Guy G., and Dirk F. E. Thys van den Audenaerde. 2003. Cychlidae. In Fresh and brackish water fishes of West Africa, ed. Didier Paugy, C. Lévêque, and Guy G. Teugels, 521–600. Institut de recherche pour le développement.
35 Lung’ayia, H. B. O. 1994. Some aspects of the reproductive biology of the Nile tilapia Oreochromis niloticus (L) in the Nyanza Gulf of Lake Victoria, Kenya. In Proceedings of the Second EEC Regional Seminar on Recent Trends of Research on Lake Victoria Fisheries, ed. E. Okemwa, E. O. Wakwabi, and A. Getabu, 121–127. Nairobi: ICIPE SCIENCE.
36 Bwanika, G. N., B. Makanga, Y. Kizito, L. J. Chapman, and J. Balirwa. 2004. Observations on the biology of Nile tilapia, Oreochromis niloticus L., in two Ugandan crater lakes. African Journal of Ecology 42: 93–101. https://doi.org/10.1111/j.1365-2028.2004.00468.x.
37 Bwanika, G. N., L. J. Chapman, Y. Kizito, and J. Balirwa. 2006. Cascading effects of introduced Nile perch (Lates niloticus) on the foraging ecology of Nile tilapia (Oreochromis niloticus). Ecology of Freshwater Fish 15: 470–481. https://doi.org/10.1111/j.1600-0633.2006.00185.x.
38 Oso, J. A., I. A. Ayodele, and O. Fagbuaro. 2006. Food and Feeding Habits of Oreochromis niloticus (L.) and Sarotherodon galilaeus (L.) in a Tropical Reservoir. World Journal of Zoology 1: 118–121.
39 Zengeya, Tsungai Alfred, Anthony J. Booth, Armanda D. S. Bastos, and Christian T. Chimimba. 2011. Trophic interrelationships between the exotic Nile tilapia, Oreochromis niloticus and indigenous tilapiine cichlids in a subtropical African river system (Limpopo River, South Africa). Environmental Biology of Fishes 92: 479–489. https://doi.org/10.1007/s10641-011-9865-4.
40 Uddin, M. S., A. Farzana, M. K. Fatema, M. E. Azim, M. A. Wahab, and M. C. J. Verdegem. 2007. Technical evaluation of tilapia (Oreochromis niloticus) monoculture and tilapia–prawn (Macrobrachium rosenbergii) polyculture in earthen ponds with or without substrates for periphyton development. Aquaculture 269: 232–240. https://doi.org/10.1016/j.aquaculture.2007.05.038.
41 Chapman, Lauren J., Colin A. Chapman, and Mark Chandler. 1996. Wetland ecotones as refugia for endangered fishes. Biological Conservation 78: 263–270. https://doi.org/10.1016/S0006-3207(96)00030-4.
42 Chapman, Lauren J., Colin A. Chapman, Richard Ogutu-Ohwayo, Mark Chandler, Les Kaufman, and Amanda E. Keiter. 1996. Refugia for Endangered Fishes from an Introduced Predator in Lake Nabugabo, Uganda. Conservation Biology 10: 554–561. https://doi.org/10.1046/j.1523-1739.1996.10020554.x.
43 Rosenberger, A. E., and L. J. Chapman. 1999. Hypoxic wetland tributaries as faunal refugia from an introduced predator. Ecology of Freshwater Fish 8: 22–34. https://doi.org/10.1111/j.1600-0633.1999.tb00049.x.
44 Schofield, Pamela J., and Lauren J. Chapman. 1999. Interactions Between Nile Perch, Lates niloticus, and Other Fishes in Lake Nabugabo, Uganda. Environmental Biology of Fishes 55: 343–358. https://doi.org/10.1023/A:1007544017989.
45 Duponchelle, Fabrice, and Marc Legendre. 2001. Rapid phenotypic changes of reproductive traits in response to experimental modifications of spatial structure in Nile tilapia, Oreochromis niloticus. Aquatic Living Resources 14: 145–152. https://doi.org/10.1016/S0990-7440(01)01109-3.
46 Delicio, Helton Carlos, Rodrigo Egydio Barreto, Edvaldo Bento Normandes, Ana Carolina Luchiari, and Ana Lúcia Marcondes. 2006. A place preference test in the fish Nile tilapia. Journal of Experimental Animal Science 43: 141–148. https://doi.org/10.1016/j.jeas.2006.01.001.
47 FAO. 2014. The State of World Fisheries and Aquaculture 2014. Rome: Food and Agriculture Organization of the United Nations.
48 Watson, R., Jackie Alder, and Daniel Pauly. 2006. Fisheries for forage fish, 1950 to the present. In On the Multiple Uses of Forage Fish: from Ecosystems to Markets, ed. Jackie Alder and Daniel Pauly, 14:1–20. Fisheries Centre Research Reports 3. Vancouver, Canada: Fisheries Centre, University of British Columbia.
49 Mood, A. 2012. Average annual fish capture for species mostly used for fishmeal (2005-2009). fishcount.org.uk.
50 Mood, A., and P. Brooke. 2012. Estimating the Number of Farmed Fish Killed in Global Aquaculture Each Year.
51 Kopf, Von Kristin. 2012. Milliarden vs. Billionen: Große Zahlen. Sprachlog.
52 Weisstein, Eric W. 2018. Milliard. Text. MathWorld - a Wolfram Web resource. http://mathworld.wolfram.com/Milliard.html. Accessed February 2.
53 Fish, G. R. 1955. The food of Tilapia in East Africa. The Uganda journal 19: 85–89.
54 Lowe-McConnell, R. H. 2000. The roles of tilapias in ecosystems. In Tilapias: Biology and Exploitation, ed. Malcolm C. M. Beveridge and Brendan J. McAndrew, 129–162. Fish and Fisheries Series 25. Springer Netherlands.
55 Moriarity, D. J. W., Johanna P. E. C. Darlington, I. G. Dunn, Christine M. Moriarty, and M. P. Tevlin. 1973. Feeding and Grazing in Lake George, Uganda. Proceedings of the Royal Society of London B: Biological Sciences 184: 299–319. https://doi.org/10.1098/rspb.1973.0050.
56 Gophen, M., P. B. O. Ochumba, U. Pollinger, and L. S. Kaufman. 1994. Nile perch (Lates niloticus) invasion in Lake Victoria (East Africa). Verhandlungen Internationale Vereinigung Limnologie 25: 856–859.
57 Balirwa, J. S. 1998. Lake Victoria wetlands and the ecology of the Nile tilapia, Oreochromis niloticus Linne. Ph.D. dissertation, Wageningen, The Netherlands: Wageningen Agricultural University.
58 Peterson, Mark S., William T. Slack, Gretchen L. Waggy, Jeremy Finley, Christa M. Woodley, and Melissa L. Partyka. 2006. Foraging in Non-Native Environments: Comparison of Nile Tilapia and Three Co-Occurring Native Centrarchids in Invaded Coastal Mississippi Watersheds. Environmental Biology of Fishes 76: 283–301. https://doi.org/10.1007/s10641-006-9033-4.
59 Azaza, M. S., M. N. Dhraief, M. M. Kraiem, and E. Baras. 2010. Influences of food particle size on growth, size heterogeneity, food intake and gastric evacuation in juvenile Nile tilapia, Oreochromis niloticus, L., 1758. Aquaculture 309: 193–202. https://doi.org/10.1016/j.aquaculture.2010.09.026.
60 Yousif, O. M. 2002. The effects of stocking density, water exchange rate, feeding frequency and grading on size hierarchy development in juvenile Nile tilapia, Oreochromis niloticus L. Emirates Journal of Food and Agriculture 14. https://doi.org/10.9755/ejfa.v14i1.4984.
61 Mustapha, Moshood Keke, Olatoyosi Taofeek Oladokun, Mubarak Mayowa Salman, Idris Adewale Adeniyi, and Dele Ojo. 2014. Does light duration (photoperiod) have an effect on the mortality and welfare of cultured Oreochromis niloticus and Clarias gariepinus? Turkish Journal of Zoology 38: 466–470.
62 Luchiari, A. C., and F. a. M. Freire. 2009. Effects of environmental colour on growth of Nile tilapia, Oreochromis niloticus (Linnaeus, 1758), maintained individually or in groups. Journal of Applied Ichthyology 25: 162–167. https://doi.org/10.1111/j.1439-0426.2008.01203.x.
63 Gilbert, P. 1996. Breeding and propagation of tilapia (Oreochromis niloticus) in a floating hatchery, Gabon. Naga, the ICLARM Quarterly 19: 26–33.
64 Azaza, M. S., M. N. Dhraief, and M. M. Kraiem. 2008. Effects of water temperature on growth and sex ratio of juvenile Nile tilapia Oreochromis niloticus (Linnaeus) reared in geothermal waters in southern Tunisia. Journal of Thermal Biology 33: 98–105. https://doi.org/10.1016/j.jtherbio.2007.05.007.
65 Philippart, J.-Cl., and J.-Cl. Ruwet. 1982. Ecology and Distribution of Tilapias. In The Biology and Culture of Tilapias: Proceedings of the International Conference on the Biology and Culture of Tilapias, 2-5 September 1980 at the Study and Conference Center of the Rockefeller Foundation, Bellagio, Italy, ed. Roger S. V. Pullin and R. H. Lowe-McConnell, 15–59. ICLARM Conference Proceedings 7. WorldFish.
66 Baroiller, Jean-Francois, Daniel Chourrout, Alexis Fostier, and Bernard Jalabert. 1995. Temperature and sex chromosomes govern sex ratios of the mouthbrooding Cichlid fish Oreochromis niloticus. Journal of Experimental Zoology 273: 216–223. https://doi.org/10.1002/jez.1402730306.
67 Abucay, Jose S, Graham C Mair, David O F Skibinski, and John A Beardmore. 1999. Environmental sex determination: the effect of temperature and salinity on sex ratio in Oreochromis niloticus L. Aquaculture 173: 219–234. https://doi.org/10.1016/S0044-8486(98)00489-X.
68 Cataldi, Emilia, Donatella Crosetti, Camilla Leoni, and Stefano Cataudella. 1988. Oesophagus structure during adaptation to salinity in oreochromis niloticus (Perciformes, pisces) juveniles. Bolletino di zoologia 55: 59–62. https://doi.org/10.1080/11250008809386600.
69 Videler, J. J. 1974. On the Interrelationships Between Morphology and Movement in the Tail of the Cichlid Fish Tilapia Nilotica (L.). Netherlands Journal of Zoology 25: 143–194. https://doi.org/10.1163/002829675X00209.
70 Lindsey, C. C. 1978. Form, function and locomotory habits in fish. In Fish Physiology VII, ed. William S. Hoar and D. J. Randall, 1–100. New York: Academic Press.
71 van Oijen, M. J. P. 1995. Appendix I. Key to Lake Victoria fishes other than haplochromine cichlids. In Fish Stocks and Fisheries of Lake Victoria: A Handbook for Field Observations, ed. Frans Witte and Wim L. T. van Densen, 209–300. Dyfed, Great Britain: Samara Publishing Limited.
72 Goudswaard, P. C., F. Witte, and E. F. B. Katunzi. 2002. The tilapiine fish stock of Lake Victoria before and after the Nile perch upsurge. Journal of Fish Biology 60: 838–856. https://doi.org/10.1111/j.1095-8649.2002.tb02413.x.
73 Fujimura, Koji, and Norihiro Okada. 2007. Development of the embryo, larva and early juvenile of Nile tilapia Oreochromis niloticus (Pisces: Cichlidae). Developmental staging system. Development, Growth & Differentiation 49: 301–324. https://doi.org/10.1111/j.1440-169X.2007.00926.x.
74 Pawson, M.G., and G.D. Pickett. 1996. The Annual Pattern of Condition and Maturity in Bass, Dicentrarchus Labrax, in Waters Around England and Wales. Journal of the Marine Biological Association of the United Kingdom 76: 107. https://doi.org/10.1017/S0025315400029040.
75 Siddiqui, A. Q., M. S. Howlader, and A. B. Adam. 1989. Culture of Nile tilapia, Oreochromis niloticus (L.), at three stocking densities in outdoor concrete tanks using drainage water. Aquaculture Research 20: 49–58. https://doi.org/10.1111/j.1365-2109.1989.tb00440.x.
76 Abou, Youssouf, Emile D Fiogbé, and Jean-Claude Micha. 2007. Effects of stocking density on growth, yield and profitability of farming Nile tilapia, Oreochromis niloticus L., fed Azolla diet, in earthen ponds. Aquaculture Research 38: 595–604. https://doi.org/10.1111/j.1365-2109.2007.01700.x.
77 Barcellos, Leonardo José Gil, S. Nicolaiewsky, S M G. De Souza, and F. Lulhier. 1999. The effects of stocking density and social interaction on acute stress response in Nile tilapia Oreochromis niloticus (L.) fingerlings. Aquaculture Research 30: 887–892. https://doi.org/10.1046/j.1365-2109.1999.00419.x.
78 Freitas, R. H. A., and G. L. Volpato. 2008. Behavioral response of Nile tilapia to an allopatric predator. Marine and Freshwater Behaviour and Physiology 41: 267–272. https://doi.org/10.1080/10236240802509767.
79 Fryer, Geoffrey, and T. D. Iles. 1972. The cichlid fishes of the great lakes of Africa: their biology and evolution. Oliver and Boyd.
80 International Game Fish Association. 2001. Database of IGFA angling records until 2001. Fort Lauderdale, USA: IGFA.
81 Lambooij, E., M. A. Gerritzen, H. Reimert, D. Burggraaf, and J. W. van de Vis. 2008. A humane protocol for electro-stunning and killing of Nile tilapia in fresh water. Aquaculture 275: 88–95. https://doi.org/10.1016/j.aquaculture.2008.01.026.
82 Oliveira Filho, Paulo Roberto Campagnoli de, Pamela Jenny Montes Girao, Mariza Pires de Melo, and Elisabete Maria Macedo Viegas. 2015. Indicators of stress in tilapia subjected to different stunning methods. Boletim do Instituto de Pesca, São Paulo 41: 335–343.
83 Tessema, Misikire, Andreas Müller-Belecke, and Gabriele Hörstgen-Schwark. 2006. Effect of rearing temperatures on the sex ratios of Oreochromis niloticus populations. Aquaculture 258: 270–277. https://doi.org/10.1016/j.aquaculture.2006.04.041.
84 Vera Cruz, E. M., and G. C. Mair. 1994. Conditions for effective androgen sex reversal in Oreochromis niloticus (L.). Aquaculture 122: 237–248. https://doi.org/10.1016/0044-8486(94)90513-4.
85 Phelps, R. P., G. Conterras Salazar, V. Abe, and B. J. Argue. 1995. Sex reversal and nursery growth of Nile tilapia, Oreochromis niloticus (L.), free-swimming in earthen ponds. Aquaculture Research 26: 293–295. https://doi.org/10.1111/j.1365-2109.1995.tb00915.x.
86 Mair, G. C., A. G. Scott, D. J. Penman, J. A. Beardmore, and D. O. F. Skibinski. 1991. Sex determination in the genus Oreochromis. Theoretical and Applied Genetics 82: 144–152. https://doi.org/10.1007/BF00226205.
87 Mair, G. C., J. S. Abucay, T. A. Abella, J. A. Beardmore, and D. O. F. Skibinski. 1997. Genetic manipulation of sex ratio for the large-scale production of all-male tilapia Oreochromis niloticus. Canadian Journal of Fisheries and Aquatic Sciences 54: 396–404. https://doi.org/10.1139/f96-282.
88 NOT FOUND
89 Shoko, Amon Paul, Samwel Mchele Limbu, Hillary Deogratias John Mrosso, Adolf Faustine Mkenda, and Yunus Daud Mgaya. 2016. Effect of stocking density on growth, production and economic benefits of mixed sex Nile tilapia (Oreochromis niloticus) and African sharptooth catfish (Clarias gariepinus) in polyculture and monoculture. Aquaculture Research 47: 36–50. https://doi.org/10.1111/are.12463.
90 Noakes, D. L. G., and E. K. Balon. 1982. Life Histories of Tilapias: An Evolutionary Perspective. In The Biology and Culture of Tilapias: Proceedings of the International Conference on the Biology and Culture of Tilapias, 2-5 September 1980 at the Study and Conference Center of the Rockefeller Foundation, Bellagio, Italy, ed. Roger S. V. Pullin and R. H. Lowe-McConnell, 61–82. ICLARM Conference Proceedings 7. WorldFish.
91 Worthington, E. B. 1932. A report on the fisheries of Uganda investigated by the Cambridge Expedition to the East African Lakes, 1930-31. Monograph or Serial Issue.
92 Fessehaye, Yonas, Zizy El-bialy, Mahmoud A. Rezk, Richard Crooijmans, Henk Bovenhuis, and Hans Komen. 2006. Mating systems and male reproductive success in Nile tilapia (Oreochromis niloticus) in breeding hapas: A microsatellite analysis. Aquaculture 256: 148–158. https://doi.org/10.1016/j.aquaculture.2006.02.024.
93 Bautista, A. M., M. H. Carlos, and A. I. San Antonio. 1988. Hatchery production of Oreochromis niloticus L. at different sex ratios and stocking densities. Aquaculture 73: 85–89. https://doi.org/10.1016/0044-8486(88)90043-9.
94 Lisney, T. J., E. Studd, and C. W. Hawryshyn. 2010. Electrophysiological assessment of spectral sensitivity in adult Nile tilapia Oreochromis niloticus: evidence for violet sensitivity. The Journal of Experimental Biology 213: 1453–1463. https://doi.org/10.1242/jeb.036897.
95 Barcellos, Leonardo José Gil, Gilson Luiz Volpato, Rodrigo Egydio Barreto, Ivanir Coldebella, and Daiane Ferreira. 2011. Chemical communication of handling stress in fish. Physiology & Behavior 103: 372–375. https://doi.org/10.1016/j.physbeh.2011.03.009.
96 Smith, Michael E., Andrew S. Kane, and Arthur N. Popper. 2004. Acoustical stress and hearing sensitivity in fishes: does the linear threshold shift hypothesis hold water? Journal of Experimental Biology 207: 3591–3602. https://doi.org/10.1242/jeb.01188.
97 Brown, Culum. 2015. Fish intelligence, sentience and ethics. Animal Cognition 18: 1–17. https://doi.org/10.1007/s10071-014-0761-0.
98 Amundsen, Lasse, and Martin Landro. 2011. Marine seismic sources part VIII: Fish hear a great deal. Recent Advances in Technology 8: 1–5.
99 Roques, Jonathan A. C., Wout Abbink, Femke Geurds, Hans van de Vis, and Gert Flik. 2010. Tailfin clipping, a painful procedure: Studies on Nile tilapia and common carp. Physiology & Behavior 101: 533–540. https://doi.org/10.1016/j.physbeh.2010.08.001.
100 Réale, Denis, Simon M. Reader, Daniel Sol, Peter T. McDougall, and Niels J. Dingemanse. 2007. Integrating animal temperament within ecology and evolution. Biological Reviews 82: 291–318. https://doi.org/10.1111/j.1469-185X.2007.00010.x.
101 Volpato, G. L., and R. E. Barreto. 2001. Environmental blue light prevents stress in the fish Nile tilapia. Brazilian Journal of Medical and Biological Research 34: 1041–1045. https://doi.org/10.1590/S0100-879X2001000800011.
102 Robb, D H F, and S C Kestin. 2002. Methods Used to Kill Fish: Field Observations and Literature Reviewed. Animal Welfare 11: 269–282.
❮
❯



«