Information
Version: B | 1.1 (2022-01-22)
WelfareScore | farm
Condensed assessment of the species' likelihood and potential for good fish welfare in aquaculture, based on ethological findings for 10 crucial criteria.
- Li = Likelihood that the individuals of the species experience good welfare under minimal farming conditions
- Po = Potential of the individuals of the species to experience good welfare under high-standard farming conditions
- Ce = Certainty of our findings in Likelihood and Potential
WelfareScore = Sum of criteria scoring "High" (max. 10)
General remarks
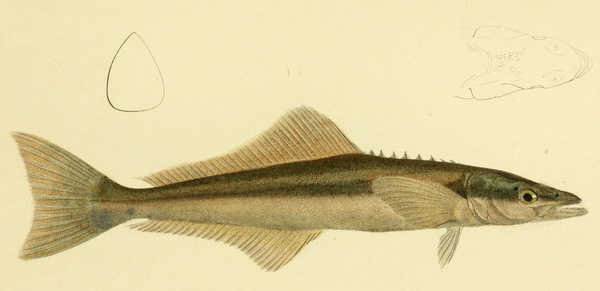
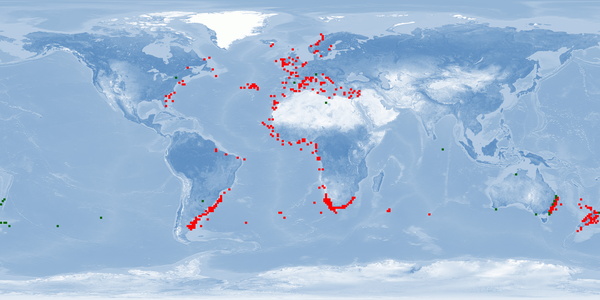
Rachycentron canadum is a migratory pelagic species widely distributed in subtropical, tropical, and temperate areas, except for the central and eastern Pacific. Several biological attributes make R. canadum a strong candidate for aquaculture, such as rapid growth, high fillet quality, and high market price. However, many aspects of welfare in rearing conditions have not been addressed yet. In order to optimise fish welfare of this species, improvements in current harvesting practices are needed to meet depth range needs, reproduction without manipulation, aggregation, aggression, and stress reduction.
1 Home range
Many species traverse in a limited horizontal space (even if just for a certain period of time per year); the home range may be described as a species' understanding of its environment (i.e., its cognitive map) for the most important resources it needs access to.
What is the probability of providing the species' whole home range in captivity?
It is unclear for minimal and high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


2 Depth range
Given the availability of resources (food, shelter) or the need to avoid predators, species spend their time within a certain depth range.
What is the probability of providing the species' whole depth range in captivity?
It is low for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a high amount of evidence.


3 Migration
Some species undergo seasonal changes of environments for different purposes (feeding, spawning, etc.), and to move there, they migrate for more or less extensive distances.
What is the probability of providing farming conditions that are compatible with the migrating or habitat-changing behaviour of the species?
It is low for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


4 Reproduction
A species reproduces at a certain age, season, and sex ratio and possibly involving courtship rituals.
What is the probability of the species reproducing naturally in captivity without manipulation of theses circumstances?
It is low for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


5 Aggregation
Species differ in the way they co-exist with conspecifics or other species from being solitary to aggregating unstructured, casually roaming in shoals or closely coordinating in schools of varying densities.
What is the probability of providing farming conditions that are compatible with the aggregation behaviour of the species?
It is low for minimal and high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


6 Aggression
There is a range of adverse reactions in species, spanning from being relatively indifferent towards others to defending valuable resources (e.g., food, territory, mates) to actively attacking opponents.
What is the probability of the species being non-aggressive and non-territorial in captivity?
It is low for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


7 Substrate
Depending on where in the water column the species lives, it differs in interacting with or relying on various substrates for feeding or covering purposes (e.g., plants, rocks and stones, sand and mud, turbidity).
What is the probability of providing the species' substrate and shelter needs in captivity?
It is low for minimal farming conditions. It is high for high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


8 Stress
Farming involves subjecting the species to diverse procedures (e.g., handling, air exposure, short-term confinement, short-term crowding, transport), sudden parameter changes or repeated disturbances (e.g., husbandry, size-grading).
What is the probability of the species not being stressed?
It is low for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


9 Malformations
Deformities that – in contrast to diseases – are commonly irreversible may indicate sub-optimal rearing conditions (e.g., mechanical stress during hatching and rearing, environmental factors unless mentioned in crit. 3, aquatic pollutants, nutritional deficiencies) or a general incompatibility of the species with being farmed.
What is the probability of the species being malformed rarely?
It is low for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a low amount of evidence.


10 Slaughter
The cornerstone for a humane treatment is that slaughter a) immediately follows stunning (i.e., while the individual is unconscious), b) happens according to a clear and reproducible set of instructions verified under farming conditions, and c) avoids pain, suffering, and distress.
What is the probability of the species being slaughtered according to a humane slaughter protocol?
It is low for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


Side note: Domestication
Teletchea and Fontaine introduced 5 domestication levels illustrating how far species are from having their life cycle closed in captivity without wild input, how long they have been reared in captivity, and whether breeding programmes are in place.
What is the species’ domestication level?
DOMESTICATION LEVEL 4 46, level 5 being fully domesticated.
Side note: Forage fish in the feed
450-1,000 milliard wild-caught fishes end up being processed into fish meal and fish oil each year which contributes to overfishing and represents enormous suffering. There is a broad range of feeding types within species reared in captivity.
To what degree may fish meal and fish oil based on forage fish be replaced by non-forage fishery components (e.g., poultry blood meal) or sustainable sources (e.g., soybean cake)?
All age classes: WILD: carnivorous 9 47. FARM: fish meal and fish oil may be partly* replaced by non-forage fishery components 48 49 50 51 52.
*partly = <51% – mostly = 51-99% – completely = 100%
Glossary
DOMESTICATION LEVEL 4 = entire life cycle closed in captivity without wild inputs 46
EURYHALINE = tolerant of a wide range of salinities
FARM = setting in farming environment or under conditions simulating farming environment in terms of size of facility or number of individuals
FINGERLINGS = early juveniles with fully developed scales and working fins, the size of a human finger; for details ➝ Findings 10.1 Ontogentic development
IND = individuals
JUVENILES = fully developed but immature individuals, for details ➝ Findings 10.1 Ontogenetic development
LAB = setting in laboratory environment
LARVAE = hatching to mouth opening, for details ➝ Findings 10.1 Ontogenetic development
OCEANODROMOUS = living and migrating in the sea
PELAGIC = living independent of bottom and shore of a body of water
PHOTOPERIOD = duration of daylight
SPAWNERS = adults during the spawning season; in farms: adults that are kept as broodstock
WILD = setting in the wild
Bibliography
2 Benetti, Daniel D., Mehmet R. Orhun, Bruno Sardenberg, Brian O’Hanlon, Aaron Welch, Ronald Hoenig, Ian Zink, et al. 2008. Advances in hatchery and grow-out technology of cobia Rachycentron canadum (Linnaeus). Aquaculture Research 39: 701–711. https://doi.org/10.1111/j.1365-2109.2008.01922.x.
3 Liao, I Chiu, Ting-Shih Huang, Wann-Sheng Tsai, Cheng-Ming Hsueh, Su-Lean Chang, and Eduardo M Leaño. 2004. Cobia culture in Taiwan: current status and problems. Aquaculture 237: 155–165. https://doi.org/10.1016/j.aquaculture.2004.03.007.
4 Benetti, D., M. R. Orhun, I. C. Zink, F. G. Cavalin, B. Sardenberg, K. Palmer, B. Denlinger, D. Bacoat, and B. O’Hanlon. 2010. Growth rates of cobia (Rachycentron canadum) cultured in open ocean submerged cages in the Caribbean. Aquaculture 302: 195–201. https://doi.org/http://dx.doi.org/10.1016/j.aquaculture.2010.02.021.
5 Nhu, Van Can, Huy Quang Nguyen, Thanh Luu Le, Mai Thien Tran, Patrick Sorgeloos, Kristof Dierckens, Helge Reinertsen, Elin Kjørsvik, and Niels Svennevig. 2011. Cobia Rachycentron canadum aquaculture in Vietnam: Recent developments and prospects. Aquaculture 315. Larvi 2009: 20–25. https://doi.org/10.1016/j.aquaculture.2010.07.024.
6 Arnold, Connie R., Jeffrey B. Kaiser, and G. Joan Holt. 2002. Spawning of Cobia Rachycentron canadum in Captivity. Journal of the World Aquaculture Society 33: 205–208. https://doi.org/10.1111/j.1749-7345.2002.tb00496.x.
7 Shaffer, Rosalie Vaught, and Eugene L. Nakamura. 1989. Synopsis of biological data on the cobia Rachycentron canadum (Pisces: Rachycentridae). U.S. Dept. of Commerce, National Oceanic and Atmospheric Administration, National Marine Fisheries Service.
8 Richards, C. E. 1977. Cobia (Rachycentron canadum) tagging within Chesapeake Bay and updating of growth equations. Chesapeake Science 18: 310–311. https://doi.org/10.2307/1350806.
9 Smith, Jospeh W. 1995. Life History of Cobia (Osteichthyes: Rachycentridae), in North Carolina Waters. Brimleyana 23: 1–23.
10 South Carolina Marine Resources Research Institute. 1995. Unpublished data. Charleston, South Carolina.
11 Springer, S., and H.R. Bullis. 1956. Collections by the Oregon in the Gulf of Mexico: list of crustaceans, mollusks, and fishes identified from collections made by the exploratory fishing vessel Oregon in the Gulf of Mexico and adjacent seas 1950 through 1955 /. Washington, D.C. :
12 Mclean, E., G. Salze, M. H. Schwarz, and S. R. Craig. 2009. Cobia cultivation in aquaculture. In New Technologies in Aquaculture, ed. Gavin Burnell and Geoff Allan, 804–821. Woodhead Publishing Series in Food Science, Technology and Nutrition. Woodhead Publishing. https://doi.org/10.1533/9781845696474.5.804.
13 Joseph, Edwin B., John J. Norcross, and William H. Massmann. 1964. Spawning of the cobia, Rachycentron canadum, in the Chesapeake Bay area, with observations of juvenile specimens. Chesapeake Science 5: 67–71. https://doi.org/10.2307/1350791.
14 Carvalho Filho, A. 1999. Peixes: Costa Brasileira. 3rd ed. São Paulo: Editora Melro Ltda.
15 Figueiredo, J.L., and N.A. Menezes. 2000. Manual de peixes marinhos do Sudeste do Brasil. VI. Teleostei. São Paulo: Museu de Zoologia, USP 1.
16 Riede, K. 2004. Global register of migratory species - from global to regional scales. Final report of the R&D Projekt 808 05 081. Bonn, Germany: Federal Agency for Nature Conservation.
17 Lefebvre, Lyndsey S., and Michael R. Denson. 2012. Inshore spawning of cobia (Rachycentron canadum) in South Carolina. Fishery Bulletin 110: 397–412.
18 Richards, C. E. 1967. Age, Growth and Fecundity of the Cobia, Rachycentron canadum, from Chesapeake Bay and Adjacent Mid-Atlantic Waters. Transactions of the American Fisheries Society 96: 343–350. https://doi.org/10.1577/1548-8659(1967)96[343:AGAFOT]2.0.CO;2.
19 Feitoza, M., R. S. Rosa, and L.A. Rocha. 2005. Ecology and Zoogeography of Deep-Reef Fishes in Northeastern Brazil. Bulletin of Marine Science 76: 725–742.
20 Bryan, D. R., K. Kilfoyle, R. G. Gilmore, and R. E. Spieler. 2012. Characterization of the mesophotic reef fish community in south Florida, USA. Journal of Applied Ichthyology 29: 108–117. https://doi.org/10.1111/j.1439-0426.2012.02055.x.
21 Biesiot, Patricia M., Robert E. Caylor, and James S. Franks. 1994. Biochemical and Histological Changes During Ovarian Development of Cobia, Rachycentron Canadum, from the Northern Gulf of Mexico. Fishery Bulletin 92: 686–696.
22 Brown-Peterson, Nancy J., Robin M. Overstreet, Jeffrey M. Lotz, James S. Franks, and Karen M. Burns. 2001. Reproductive Biology of Cobia, Rachycentron canadum, from Coastal Waters of the Southern United States. Fishery Bulletin 99: 15.
23 Rickards, W.L. 2000. Sustainable Cobia Culture and Fisheries. Vol. VSG-01-07. Charlottesville, Virginia: Virginia Sea Grant Publication.
24 Kilduff, P., W. DuPaul, M. Oesterling, J. Jr. Olney, and J. Tellock. 2002. Induced tank spawning of cobia, Rachycentron canadum, and early larval husbandry. World Aquaculture 33: 35–39.
25 Caylor, Robert E, Patricia M Biesiot, and James S Franks. 1994. Culture of cobia (Rachycentron canadum): cryopreservation of sperm and induced spawning. Aquaculture 125: 81–92. https://doi.org/10.1016/0044-8486(94)90285-2.
26 Benson, Norman Gustaf. 1982. Life History Requirements of Selected Finfish and Shellfish in Mississippi Sound and Adjacent Areas. Fish and Wildlife Service.
27 Faulk, Cynthia K., Jeffrey B. Kaiser, and G. Joan Holt. 2007. Growth and survival of larval and juvenile cobia Rachycentron canadum in a recirculating raceway system. Aquaculture 270: 149–157. https://doi.org/10.1016/j.aquaculture.2007.03.029.
28 Salze, G., E. McLean, M. H. Schwarz, and S. R. Craig. 2008. Dietary mannan oligosaccharide enhances salinity tolerance and gut development of larval cobia. Aquaculture 274: 148–152. https://doi.org/10.1016/j.aquaculture.2007.11.008.
29 Holt, G. Joan, J. Kaiser, and C. Faulk. 2007. Advances in Cobia Research in Texas. In Cobia Aquaculture: Research, Development and Commercial Production. New Orleans, La, USA: Liao and Leano, Eds World Aquaculture Society.
30 Webb, Kenneth A., Glenn M. Hitzfelder, Cynthia K. Faulk, and G. Joan Holt. 2007. Growth of juvenile cobia, Rachycentron canadum, at three different densities in a recirculating aquaculture system. Aquaculture 264: 223–227. https://doi.org/10.1016/j.aquaculture.2006.12.029.
31 Costa-Bomfim, C. N., W. V. N. Pessoa, R. L. M. Oliveira, J. L. Farias, E. C. Domingues, S. Hamilton, and R. O. Cavalli. 2014. The effect of feeding frequency on growth performance of juvenile cobia, Rachycentron canadum (Linnaeus, 1766). Journal of Applied Ichthyology 30: 135–139. https://doi.org/10.1111/jai.12339.
32 Weirich, Charles R., Alvin D. Stokes, Theodore I. J. Smith, Wallace E. Jenkins, and Michael R. Denson. 2006. Outdoor Tank and Pond Spawning of Cobia, Rachycentron canadumin Coastal South Carolina. Journal of Applied Aquaculture 18: 1–16. https://doi.org/10.1300/J028v18n03_01.
33 Kaiser, J. B. 2004. Personal communication.
34 Cnaani, Avner, and Ewen McLean. 2009. Time-course response of cobia (Rachycentron canadum) to acute stress. Aquaculture 289: 140–142. https://doi.org/10.1016/j.aquaculture.2008.12.016.
35 Trushenski, J., M. Schwarz, R. Takeuchi, B. Delbos, and L. A. Sampaio. 2010. Physiological responses of cobia Rachycentron canadum following exposure to low water and air exposure stress challenges. Aquaculture 307: 173–177. https://doi.org/10.1016/j.aquaculture.2010.07.015.
36 Rodrigues, Ricardo Vieira, Janaína dos Santos Pedron, Luis Alberto Romano, Marcelo Borges Tesser, and Luís André Sampaio. 2015. Acute responses of juvenile cobia Rachycentron canadum (Linnaeus 1766) to acid stress. Aquaculture Research 46: 1241–1247. https://doi.org/10.1111/are.12282.
37 Pedron, Janaína S., Denise S. Miron, Ricardo V. Rodrigues, and Marcelo H. Okamoto. 2016. Stress response in transport of juvenile cobia Rachycentron canadum using the anesthetic benzocaine. Latin American Journal of Aquatic Research 44: 638–642.
38 Chu, Kua Beng, Azila Abdulah, Siti Zahrah Abdullah, and Ramley Abu Bakar. 2013. A Case Study on the Mortality of Cobia (Rachycentron canadum) Cultured in Traditional Cages. Tropical Life Sciences Research 24: 77–84.
39 Moraes, J.R.E., J.R. Engracia Filho, F.R. de Moraes, C.E. Kerber, B. Plastina, and M. Shimada. 2013. Deformities in reared cobia, Rachycentron canadum L. and grouper, Epinephelus marginatus, in Sao Paulo state coast, Brazil. In Aquaculture Europe 2013. Trondheim, Norway.
40 Dutney, Luke. 2016. Reproductive Biology and Controlled Reproductive Development of Captive Cobia (Rachycentron canadum). Doctor of Philosophy, Sippy Downs, QLD, Australia: University of the Sunshine Coast.
41 Erikson, U., and N. Svennevig. 2009. A Review of Harvesting and Post-Harvesting Procedures of Marine Fish in Cage Culture with Specific Reference to Cobia Compared with Atlantic Salmon. In Proceedings of the Second Int. Symp. on Cage Aquaculture in Asia. Asian Fisheries Society, Manulla, Philippines and Zhejiang University, Hangzhou, China.
42 Trushenski, Jesse T., John C. Bowzer, James D. Bowker, and Michael H. Schwarz. 2012. Chemical and Electrical Approaches to Sedation of Cobia: Induction, Recovery, and Physiological Responses to Sedation. Marine and Coastal Fisheries 4: 639–650. https://doi.org/10.1080/19425120.2012.728182.
43 Vargas Baldi, Sheyla Cristina, Giuliana Parisi, Antonio Bonelli, Júlio Cesar Carvalho Balieiro, Judite Lapa Guimarães, and Elisabete Maria Macedo Viegas. 2018. Effects of different stunning/slaughter methods on frozen fillets quality of cobia (Rachycentron canadum). Aquaculture 486: 107–113. https://doi.org/10.1016/j.aquaculture.2017.12.003.
44 Melo, Fúlvio Viegas Santos Teixeira de, Elisabete Maria Macedo Viegas, Giuliana Parisi, Adriana Cristina Bordignon, Manoel Adriano da Cruz Neto, and Jose Fernando Bibiano Melo. 2019. Physical, chemical and sensory evaluation of meat from cobia (rachycentron canadum), desensitized with different voltages of electric shock, stored under refrigeration. Ciência Rural 49. https://doi.org/10.1590/0103-8478cr20180242.
45 Viegas, E. M. M., P. J. M. Girão, S. C. Vargas, P. R. C. Oliveira Filho, and M. P. Melo. 2013. Interactions between slaughtering methods and stress levels of bijupira (Rachycentron canadum). In Aquaculture Europe 2013. Trondheim, Norway.
46 Teletchea, Fabrice, and Pascal Fontaine. 2012. Levels of domestication in fish: implications for the sustainable future of aquaculture. Fish and Fisheries 15: 181–195. https://doi.org/10.1111/faf.12006.
47 Arendt, M.D., J.E. Olney, and J.A. Lucy. 2001. Stomach content analysis of cobia, Rachycentron canadum, from lower Chesapeake Bay. Fishery Bulletin 99: 665–670.
48 Chou, R. L., B. Y. Her, M. S. Su, G. Hwang, Y. H. Wu, and H. Y. Chen. 2004. Substituting fish meal with soybean meal in diets of juvenile cobia Rachycentron canadum. Aquaculture 229: 325–333. https://doi.org/10.1016/S0044-8486(03)00395-8.
49 Lunger, Angela N., S. R. Craig, and E. McLean. 2006. Replacement of fish meal in cobia (Rachycentron canadum) diets using an organically certified protein. Aquaculture 257: 393–399. https://doi.org/10.1016/j.aquaculture.2005.11.010.
50 Lunger, Angela N., E. McLean, and S. R. Craig. 2007. The effects of organic protein supplementation upon growth, feed conversion and texture quality parameters of juvenile cobia (Rachycentron canadum). Aquaculture 264: 342–352. https://doi.org/10.1016/j.aquaculture.2006.12.012.
51 Silva Júnior, R. F., W. V. Nova, J. L. Farias, C. N. Costa-Bomfim, M. B. Tesser, J. I. Druzian, E. S. Correia, and R. O. Cavalli. 2011. Replacement of fish oil by soybean oil in diets for cobia (Rachycentron canadum). Arquivo Brasileiro de Medicina Veterinária e Zootecnia 63: 980–987. https://doi.org/10.1590/S0102-09352011000400025.
52 Felix, N., S. Kalaivani, U.B. Murugan, and K. Rajaram. 2014. Replacement of fish meal in cobia (Rachycentron canadum) diet with squid waste and squid waste silage. International Journal of Fisheries and Aquatic Studies 1: 256–260.