Information
Version: B | 1.2 (2022-07-20)
WelfareScore | farm
Condensed assessment of the species' likelihood and potential for good fish welfare in aquaculture, based on ethological findings for 10 crucial criteria.
- Li = Likelihood that the individuals of the species experience good welfare under minimal farming conditions
- Po = Potential of the individuals of the species to experience good welfare under high-standard farming conditions
- Ce = Certainty of our findings in Likelihood and Potential
WelfareScore = Sum of criteria scoring "High" (max. 10)
General remarks
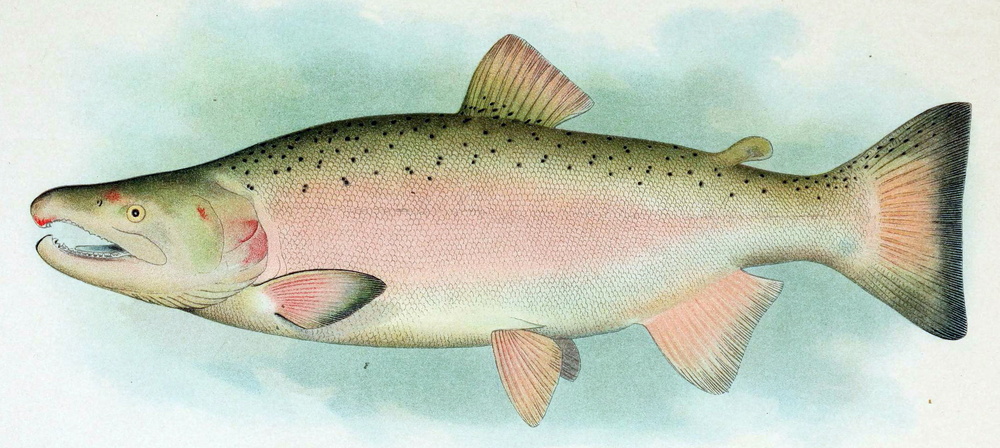
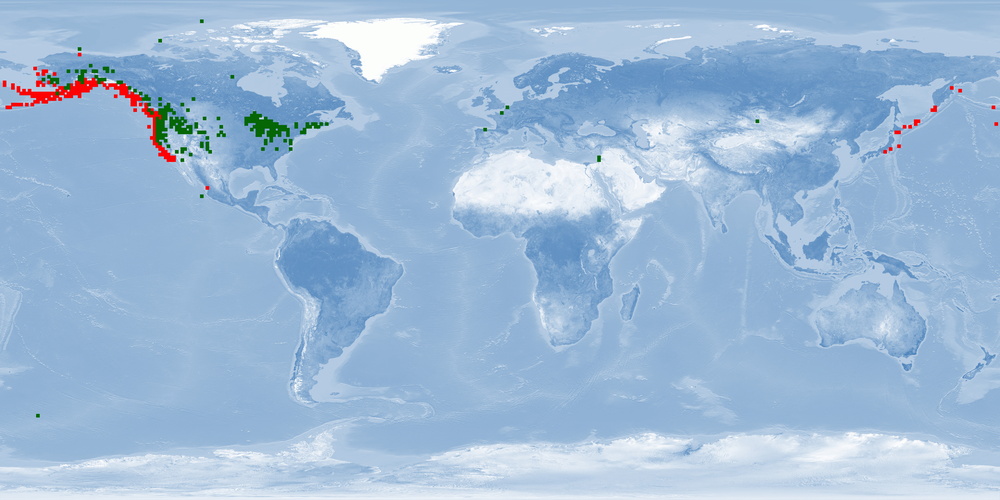
Oncorhynchus kisutch is a Pacific salmon species distributed in the North Pacific basins from northern Japan through Kamchatka, across the Bering Sea to Alaska, and south through all coastal areas to California. O. kisutch has been introduced into many areas of North America, Asia, Europe, and South America. There are two strains, an anadromous one that migrates and a resident one that stays in freshwater lakes. Within the migratory strain, two morphotypes have been described, an "ocean/coastal" type with small median fins and more streamlined body that lives offshore and an "inshore/interior" type with large median fins and a robust body that remains within inside waters. In anadromous O. kisutch: eggs hatch in streams, juveniles (parr) live in streams for one year and metamorphose into smolts that will migrate to the ocean. In the ocean, smolts grow into adults and might migrate up north. When they are close to maturity, they migrate back to their original streams to spawn. Females create several nests in a defended area called redd. O. kisutch dies after reproduction. In some farms, only females are raised, which are less aggressive. Some farms have an accelerated cycle for maximising growth and reducing the hatchery cycle by 9 months: they rear them in fresh water until parr/smolt, then place them in salt water (during peak of PHOTOPERIOD - July) until harvest, which occurs at around 19 months of age at 2.5-3.5 kg. Because of their need to migrate as adults, it is unlikely that current farms can provide this welfare need. Further research needs to be done to accommodate this need into farming conditions and on living offshore (home range, aggression, substrate). Currently, the main producer is Chile, with some smaller production in Japan, Canada and the USA. O. kisutch will be affected by climate change.
1 Home range
Many species traverse in a limited horizontal space (even if just for a certain period of time per year); the home range may be described as a species' understanding of its environment (i.e., its cognitive map) for the most important resources it needs access to.
What is the probability of providing the species' whole home range in captivity?
It is low for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a low amount of evidence.


2 Depth range
Given the availability of resources (food, shelter) or the need to avoid predators, species spend their time within a certain depth range.
What is the probability of providing the species' whole depth range in captivity?
It is low for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


3 Migration
Some species undergo seasonal changes of environments for different purposes (feeding, spawning, etc.), and to move there, they migrate for more or less extensive distances.
What is the probability of providing farming conditions that are compatible with the migrating or habitat-changing behaviour of the species?
It is low for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


4 Reproduction
A species reproduces at a certain age, season, and sex ratio and possibly involving courtship rituals.
What is the probability of the species reproducing naturally in captivity without manipulation of theses circumstances?
It is low for minimal and high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


5 Aggregation
Species differ in the way they co-exist with conspecifics or other species from being solitary to aggregating unstructured, casually roaming in shoals or closely coordinating in schools of varying densities.
What is the probability of providing farming conditions that are compatible with the aggregation behaviour of the species?
It is low for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


6 Aggression
There is a range of adverse reactions in species, spanning from being relatively indifferent towards others to defending valuable resources (e.g., food, territory, mates) to actively attacking opponents.
What is the probability of the species being non-aggressive and non-territorial in captivity?
It is low for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


7 Substrate
Depending on where in the water column the species lives, it differs in interacting with or relying on various substrates for feeding or covering purposes (e.g., plants, rocks and stones, sand and mud, turbidity).
What is the probability of providing the species' substrate and shelter needs in captivity?
It is low for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


8 Stress
Farming involves subjecting the species to diverse procedures (e.g., handling, air exposure, short-term confinement, short-term crowding, transport), sudden parameter changes or repeated disturbances (e.g., husbandry, size-grading).
What is the probability of the species not being stressed?
It is low for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


9 Malformations
Deformities that – in contrast to diseases – are commonly irreversible may indicate sub-optimal rearing conditions (e.g., mechanical stress during hatching and rearing, environmental factors unless mentioned in crit. 3, aquatic pollutants, nutritional deficiencies) or a general incompatibility of the species with being farmed.
What is the probability of the species being malformed rarely?
It is unclear for minimal and high-standard farming conditions. Our conclusion is based on a low amount of evidence.


10 Slaughter
The cornerstone for a humane treatment is that slaughter a) immediately follows stunning (i.e., while the individual is unconscious), b) happens according to a clear and reproducible set of instructions verified under farming conditions, and c) avoids pain, suffering, and distress.
What is the probability of the species being slaughtered according to a humane slaughter protocol?
It is low for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


Side note: Domestication
Teletchea and Fontaine introduced 5 domestication levels illustrating how far species are from having their life cycle closed in captivity without wild input, how long they have been reared in captivity, and whether breeding programmes are in place.
What is the species’ domestication level?
DOMESTICATION LEVEL 5 83, fully domesticated.
Side note: Forage fish in the feed
450-1,000 milliard wild-caught fishes end up being processed into fish meal and fish oil each year which contributes to overfishing and represents enormous suffering. There is a broad range of feeding types within species reared in captivity.
To what degree may fish meal and fish oil based on forage fish be replaced by non-forage fishery components (e.g., poultry blood meal) or sustainable sources (e.g., soybean cake)?
All age classes: WILD: carnivorous 35 84 85 86 87. FARM: for PARR in fresh water 88 and for SMOLTS in sea water 89, fish meal may be partly* replaced by sustainable sources; for PARR in fresh water, fish oil may be partly* 90 to mostly* replaced 91.
* partly = <51% – mostly = 51-99% – completely = 100%
Glossary
ALEVINS = larvae until the end of yolk sac absorption, for details ➝ Findings 10.1 Ontogenetic development
ANADROMOUS = migrating from the sea into fresh water to spawn
BENTHIC = living at the bottom of a body of water, able to rest on the floor
DOMESTICATION LEVEL 5 = selective breeding programmes are used focusing on specific goals 83
FARM = setting in farming environment or under conditions simulating farming environment in terms of size of facility or number of individuals
FRY = larvae from external feeding on, for details ➝ Findings 10.1 Ontogenetic development
IND = individuals
LAB = setting in laboratory environment
PARR = juvenile stage in rivers, for details ➝ Findings 10.1 Ontogenetic development
PHOTOPERIOD = duration of daylight
SMOLTS = juvenile stage migrating to the sea, for details ➝ Findings 10.1 Ontogenetic development
SPAWNERS = adults during the spawning season; in farms: adults that are kept as broodstock
WILD = setting in the wild
Bibliography
2 Sandercock, F. K. 1991. Life history of coho salmon (Oncorhynchus kisutch). In Pacific Salmon Life Histories, ed. G. Groot, 397–446. UBC Press.
3 Dill, Lawrence M., Ronald C. Ydenberg, and Alex H. G. Fraser. 1981. Food abundance and territory size in juvenile coho salmon (Oncorhynchus kisutch). Canadian Journal of Zoology. Ottawa, Canada. https://doi.org/10.1139/z81-247.
4 Fagerlund, U. H. M., J. R. McBride, and E. T. Stone. 1981. Stress-related effects of hatchery rearing density on coho salmon. Transactions of the American Fisheries Society 110: 644–649. https://doi.org/10.1577/1548-8659(1981)110<644:SEOHRD>2.0.CO;2.
5 Sigler, John W., T. C. Bjornn, and Fred H. Everest. 1984. Effects of Chronic Turbidity on Density and Growth of Steelheads and Coho Salmon. Transactions of the American Fisheries Society 113: 142–150. https://doi.org/https://doi.org/10.1577/1548-8659(1984)113<142:EOCTOD>2.0.CO;2.
6 LocalCoho Farms. 2021. Personal communication.
7 McMahon, T. E. 1983. Habitat suitability index models: coho salmon. US Fish and Wildlife Service FWS/OBS 82: 29.
8 Semiahmoo Fish & Game Club. 2020. Salmon and Trout hatchery. SFGC.
9 Kanaka Education & Environmental Partnership Society. 2020. Bell-Irving Hatchery. KEEPS.
10 Viechnicki, Joe. 2020. Hatchery board to revisit Crystal Lake king salmon in March. KFSK.
11 Novotny, Anthony J. 1975. Net-Pen Culture of Pacific Salmon in Marine Waters. Marine Fisheries Review 37: 12.
12 Hershberger, W.K., J.M. Myers, R.N. Iwamoto, W.C. Mcauley, and A.M. Saxton. 1990. Genetic changes in the growth of coho salmon (Oncorhynchus kisutch) in marine net-pens, produced by ten years of selection. Aquaculture 85: 187–197. https://doi.org/10.1016/0044-8486(90)90018-I.
13 Fairgrieve, W. 2009. Cultured Aquatic Species Information Programme. Oncorhynchus kisutch. Rome: FAO Fisheries and Aquaculture Department.
14 West Creek Aquaculture. 2020. West Creek Aquaculture land raised farmed salmon in BC. west-creek.
15 Gribanov, V. I. 1948. The coho salmon (Onchorhynchus kisuts Walb.)-a biological sketch. Izv. Tikhookean. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr 28: 43–101.
16 NOT FOUND
17 Chapman, D. W. 1962. Aggressive Behavior in Juvenile Coho Salmon as a Cause of Emigration. Fish. Res. Bd. Canada 19: 1047–1080.
18 Mason, J. C., and D. W. Chapman. 1965. Significance of Early Emergence, Environmental Rearing Capacity, and Behavioral Ecology of Juvenile Coho Salmon in Stream Channels. Journal of the Fisheries Research Board of Canada 22: 173–190. https://doi.org/10.1139/f65-015.
19 Narver, D. W. 1978. Ecology of juvenile coho salmon: can we use present knowledge for stream enhancement. In BG Shepherd and RMJ Ginetz (rapps.) Proceedings of the 1977 Northeast Pacific Chinook and Coho Salmon Workshop. Fisheries and Marine Service (Canada) Technical Report, 759:164.
20 Trudel, M., S. Tucker, J. F. T. Morris, D. A. Higgs, and D. W. Welch. 2005. Indicators of Energetic Status in Juvenile Coho Salmon and Chinook Salmon. North American Journal of Fisheries Management 25: 374–390. https://doi.org/10.1577/M04-018.1.
21 Argue, Alexander W. 1970. Study of factors affecting exploitation of Pacific salmon in the Canadian gantlet fishery of Juan de Fuca Strait. University of British Columbia. https://doi.org/10.14288/1.0102106.
22 Royce, William F., Lynwood S. Smith, and A. C. Hart. 1968. Models of oceanic migrations of Pacific salmon and comments on guidance mechanisms. Fish Bull Fish Wildl Serv 66: 441–462.
23 Milne, Donald Johnston. 1964. The chinook and coho salmon fisheries of British Columbia. 142. ottawa: queen’s printer.
24 Briggs, John C. 1953. The behavior and reproduction of salmonid fishes in a small coastal stream. State of California, Department of Fish and Game, Marine Fisheries Branch.
25 Fleming, Ian A., and Mart R. Gross. 1992. Reproductive behavior of hatchery and wild coho salmon (Oncorhynchus kisutch): does it differ? Aquaculture 103: 101–121. https://doi.org/10.1016/0044-8486(92)90405-A.
26 Foerster, R. E., and W. E. Ricker. 1953. The coho salmon of Cultus Lake and Sweltzer Creek. Journal of the Fisheries Board of Canada 10: 293–319.
27 Davidson, Frederick Alexander, and Samuel J. Hutchinson. 1938. The geographic distribution and environmental limitations of the Pacific salmon (genus Oncorhynchus). US Government Printing Office.
28 Godfrey, Harold. 1965. Coho salmon in offshore waters. Salmon of the North Pacific Ocean. Part IX. Coho, chinook, and masu salmon in offshore waters. International North Pacific Fisheries Comm. Bulletin 16: 1–39.
29 Weisbart, Melvin. 1968. Osmotic and ionic regulation in embryos, alevins, and fry of the five species of Pacific salmon. Canadian Journal of Zoology 46: 385–397.
30 Drucker, Benson. 1972. Some life history characteristics of coho salmon of the Karluk River system, Kodiak Island, Alaska. Fishery Bulletin 70: 79–94.
31 Ebersole, Joseph L., Parker J. Wigington Jr, Joan P. Baker, Michael A. Cairns, M. Robbins Church, Bruce P. Hansen, Bruce A. Miller, Henry R. LaVigne, Jana E. Compton, and Scott G. Leibowitz. 2006. Juvenile Coho Salmon Growth and Survival across Stream Network Seasonal Habitats. Transactions of the American Fisheries Society 135: 1681–1697. https://doi.org/10.1577/T05-144.1.
32 Tripp, Derek, and Peter J. McCart. 1983. Effects of different coho stocking strategies on coho and cutthroat trout production in isolated headwater streams. Salmonid Enhancement Program, Department of Fisheries and Oceans.
33 Scarlett, W. J., and C. J. Cederholm. 1984. Juvenilecoho salmon fall-winter utilization of two small tribu.
34 Otto, Robert G., and John E. McInerney. 1970. Development of salinity preference in pre-smolt coho salmon, Oncorhynchus kisutch. Journal of the Fisheries Board of Canada 27: 793–800.
35 Shapovalov, Leo, and Alan C. Taft. 1954. The Life Histories of the Steelhead Rainbow Trout (Salmo gairdneri gairdneri) and Silver Salmon (Oncorhynchus kisutch) With Special Reference to Waddell Creek, California, and Recommendations Regarding Their Management. Fish Bulletin 98. State of California Department of Fish and Game.
36 Fraser, F. J., Edward Alfred Perry, and D. T. Lightly. 1983. Big Qualicum River Salmon Development Project: A Biological Assessment, 1959-1972. Vol. 1189. Canadian Technical Report of Fisheries and Aquatic SciencesDepartment of Fisheries and Oceans.
37 Chamberlain, Frederick M. 1907. Some observations on salmon and trout in Alaska. 627. US Government Printing Office.
38 Clemens, Wilbert Amie. 1930. Pacific Salmon Migration: the Tagging of the Coho Salmon on the East Coast of Vancouver Island in 1927 and 1928. Biological Board of Canada.
39 Morris, John F T, Marc Trudel, Mary E Thiess, Ruston M Sweeting, Joseph Fisher, Susan A Hinton, Emily A Fergusson, Joseph A Orsi, Edward V Farley, and David W Welch. 2007. Stock-Specific Migrations of Juvenile Coho Salmon Derived from Coded-Wire Tag Recoveries on the Continental Shelf of Western North America: 25.
40 Hoar, W.S. 1958. The evolution of migratory behaviour among juvenile salmon of the genus Oncorhynchus. Fish. Res. Bd Can: 15–391.
41 Van Hyning, Jack M. 1951. The ocean salmon troll fishery of Oregon.
42 Bryan, James Ernest. 1973. The influence of pipeline development on freshwater fishery resources of northern Yukon Territory: aspects of research conducted in 1971 and 1972. Information Canada.
43 Gall, Graham A.E, and Roberto Neira. 2004. Genetic analysis of female reproduction traits of farmed coho salmon (Oncorhyncus kisutch). Aquaculture 234: 143–154. https://doi.org/10.1016/j.aquaculture.2004.01.029.
44 Logan, S. M. 1967. Silver salmon studies in the Resurrection Bay area. Prog. Rep. Alaska Dep. Fish Game Sport Fish Div 9: 83–102.
45 Andersen, Bruce C. 1975. Fish Populations of Carnation Creek and Other Barkley Sound Streams, 1974: Data Record and Progress Report. Fisheries Research Board.
46 McPhail, J. D., and C. C. Lindsey. 1970. Freshwater fishes of northwestern Canada and Alaska. Fish Res Board Can 173: 1–381.
47 Robertson, O. H., Marcus A. Krupp, Sydney F. Thomas, Cutting B. Favour, Satoshi Hane, and B. C. Wexler. 1961. Hyperadrenocorticism in spawning migratory and nonmigratory rainbow trout (Salmo gairdnerii); comparison with Pacific salmon (genus Oncorhynchus). General and comparative endocrinology 1: 473–484.
48 Willis, R. A. 1954. The length of time that silver salmon spent before death on spawning grounds at Spring Creek, Wilson River in 1951-52. Fish Commission of Oregon Research Briefs 5: 27–31.
49 Thériault, Véronique, Gregory R. Moyer, Laura S. Jackson, Michael S. Blouin, and Michael A. Banks. 2011. Reduced reproductive success of hatchery coho salmon in the wild: insights into most likely mechanisms. Molecular Ecology 20: 1860–1869. https://doi.org/https://doi.org/10.1111/j.1365-294X.2011.05058.x.
50 Jalabert, Bernard, Frederick W. Goetz, Bernard Breton, Alexis Fostier, and Edward M. Donaldson. 1978. Precocious Induction of Oocyte Maturation and Ovulation in Coho Salmon, Oncorhynchus kisutch. Journal of the Fisheries Research Board of Canada 35: 1423–1429. https://doi.org/10.1139/f78-224.
51 Donaldson, Edward M., George A. Hunter, and Helen M. Dye. 1981. Induced ovulation in coho salmon (Oncorhynchus kisutch). II. Preliminary study of the use of LH-RH and two high potency LH-RH analogues. Aquaculture 26: 129–141. https://doi.org/10.1016/0044-8486(81)90116-2.
52 Hunter, George A., Edward M. Donaldson, and Helen M. Dye. 1981. Induced ovulation in coho salmon (Oncorhynchus kisutch). I. Further studies on the use of salmon pituitary preparations. Aquaculture 26: 117–127. https://doi.org/10.1016/0044-8486(81)90115-0.
53 Mundie, J. H. 1969. Ecological implications of the diet of juvenile coho salmon in streams. In Symposium on salmon and trout in streams, 135–152. University of British Columbia.
54 Hartman, G. F. 1965. The Role of Behavior in the Ecology and Interaction of Underyearling Coho Salmon (Oncorhynchus kisutch) and Steelhead Trout (Salmo gairdneri). Journal of the Fisheries Board of Canada. Ottawa, Canada. https://doi.org/10.1139/f65-095.
55 Lister, D. B., and C. E. Walker. 1966. The effect of flow control on freshwater survival of chum, coho, and chinook salmon in the Big Qualicum River. Canadian Fish Culturist 37: 3–26.
56 Chapman, D. W., and T. C. Bjornn. 1969. Distribution of salmonids in streams. In Symp. Salmon Trout Streams. Institute of Fisheries, University of British Columbia, Vancouver, 153–176.
57 Armstrong, R. W., and A. W. Argue. 1977. Trapping and coded-wire tagging of wild coho and chinook juveniles from the Cowichan River system, 1975. Fish. Mar. Serv.(Can.) Pac. Reg. Tech. Rep. Ser. PAC.
58 Ejike, Chiweyite, and Carl B. Schreck. 1980. Stress and social hierarchy rank in coho salmon. Transactions of the American Fisheries Society 109: 423–426. https://doi.org/https://doi.org/10.1577/1548-8659(1980)109<423:SASHRI>2.0.CO;2.
59 Tagart, J. V. 1984. Coho salmon survival from egg deposition to emergence. In Proceedings of the Olympic Wild Fish Conference, 1984. Peninsula College, Fisheries Technology Program.
60 Dill, Lawrenc M. 1969. The sub-gravel behaviour of Pacific salmon larvae. In Symposium on Salmon and Trout in Streams. HR MacMillan Lectures in Fisheries. Institute of Fisheries, University of British Columbia, Vancouver, 89–99.
61 Scrivener, J. C., and B. C. Andersen. 1984. Logging impacts and some mechanisms that determine the size of spring and summer populations of coho salmon fry (Oncorhynchus kisutch) in Carnation Creek, British Columbia. Canadian Journal of Fisheries and Aquatic Sciences 41: 1097–1105.
62 Lister, D. B., and H. S. Genoe. 1970. Stream habitat utilization by cohabiting underyearlings of chinook (Oncorhynchus tshawytscha) and coho (O. kisutch) salmon in the Big Qualicum River, British Columbia. Journal of the Fisheries Board of Canada 27: 1215–1224.
63 Fuss, Howard J, and Charles Johnson. 1988. Effects of Artificial Substrate and Covering on Growth and Survival of Hatchery-Reared Coho Salmon. The Progressive Fish-Culturist 50: 232–237.
64 Phillips, Robert W., Richard L. Lantz, Errol W. Claire, and John R. Moring. 1975. Some effects of gravel mixtures on emergence of coho salmon and steelhead trout fry. Transactions of the American Fisheries Society 104: 461–466. https://doi.org/10.1577/1548-8659(1975)104<461:SEOGMO>2.0.CO;2.
65 Peterson, N. Phil. 1980. The role of spring ponds in the winter ecology and natural production of coho salmon (Oncorhynchus kisutch) on the Olympic Peninsula, Washington. PhD Thesis, University of Washington.
66 Bustard, David R., and David W. Narver. 1975. Preferences of juvenile coho salmon (Oncorhynchus kisutch) and cutthroat trout (Salmo clarki) relative to simulated alteration of winter habitat. Journal of the Fisheries Board of Canada 32: 681–687.
67 Quinn, T. P., and N. P. Peterson. 2011. The influence of habitat complexity and fish size on over-winter survival and growth of individually marked juvenile coho salmon (Oncorhynchus kisutch) in Big Beef Creek, Washington. Canadian Journal of Fisheries and Aquatic Sciences. Ottawa, Canada. https://doi.org/10.1139/f96-092.
68 Noggle, C. 1977. The Behavioural and Physiological Effects of Suspended Sediment on Juvenile Salmonids. In Proc. Fourth Annual Aquatic Toxicity Workshop, 8–10.
69 Wigington, P. J., J. L. Ebersole, M. E. Colvin, S. G. Leibowitz, B. Miller, B. Hansen, H. R. Lavigne, et al. 2006. Coho salmon dependence on intermittent streams. Frontiers in Ecology and the Environment 4: 513–518. https://doi.org/https://doi.org/10.1890/1540-9295(2006)4[513:CSDOIS]2.0.CO;2.
70 Chapman, D. W., and Eric Knudsen. 1980. Channelization and livestock impacts on salmonid habitat and biomass in western Washington. Transactions of the American Fisheries Society 109: 357–363.
71 Bisson, Peter A., and Robert E. Bilby. 1982. Avoidance of Suspended Sediment by Juvenile Coho Salmon. North American Journal of Fisheries Management 2: 371–374. https://doi.org/https://doi.org/10.1577/1548-8659(1982)2<371:AOSSBJ>2.0.CO;2.
72 Pritchard, A. L. 1940. Studies on the age of the coho salmon (Oncorhynchus kisutch) and the spring salmon (Oncorhynchus tschawytscha) in British Columbia. Transactions of the Royal Society of Canada 34: 99–120.
73 Specker, J. L., and C. B. Schreck. 1980. Stress Responses to Transportation and Fitness for Marine Survival in Coho Salmon ( Oncorhynchus kisutch ) Smolts. Canadian Journal of Fisheries and Aquatic Sciences 37: 765–769. https://doi.org/10.1139/f80-102.
74 Schreck, Carl B., Mario F. Solazzi, Steven L. Johnson, and Thomas E. Nickelson. 1989. Transportation stress affects performance of coho salmon, Oncorhynchus kisutch. Aquaculture 82. Salmonid Smoltification III: 15–20. https://doi.org/10.1016/0044-8486(89)90391-8.
75 Davis, Lawrence E., and Carl B. Schreck. 1997. The Energetic Response to Handling Stress in Juvenile Coho Salmon. Transactions of the American Fisheries Society 126: 248–258. https://doi.org/10.1577/1548-8659(1997)126<0248:TERTHS>2.3.CO;2.
76 Barton, B. A., C. B. Schreck, R. D. Ewing, A. R. Hemmingsen, and R. Patiño. 1985. Changes in plasma cortisol during stress and smoltification in Coho Salmon, Oncorhynchus kisutch. General and Comparative Endocrinology 59: 468–471. https://doi.org/10.1016/0016-6480(85)90406-X.
77 Neira, Roberto, Jean Paul Lhorente, Cristian Araneda, Nelson Díaz, Eduardo Bustos, and Alejandro Alert. 2004. Studies on carcass quality traits in two populations of Coho salmon (Oncorhynchus kisutch): phenotypic and genetic parameters. Aquaculture 241: 117–131. https://doi.org/10.1016/j.aquaculture.2004.08.009.
78 Robb, D H F, and S C Kestin. 2002. Methods Used to Kill Fish: Field Observations and Literature Reviewed. Animal Welfare 11: 269–282.
79 Lines, J. A., D. H. Robb, S. C. Kestin, S. C. Crook, and T. Benson. 2003. Electric stunning: a humane slaughter method for trout. Aquacultural Engineering 28: 141–154. https://doi.org/10.1016/S0144-8609(03)00021-9.
80 European Food Safety Authority (EFSA). 2009. Species-specific welfare aspects of the main systems of stunning and killing of farmed fish: Rainbow Trout. EFSA Journal 7: 1012. https://doi.org/10.2903/j.efsa.2009.1012.
81 Concollato, Anna, Rolf Erik Olsen, Sheyla Cristina Vargas, Antonio Bonelli, Marco Cullere, and Giuliana Parisi. 2016. Effects of stunning/slaughtering methods in rainbow trout (Oncorhynchus mykiss) from death until rigor mortis resolution. Aquaculture 464: 74–79. https://doi.org/10.1016/j.aquaculture.2016.06.009.
82 Humane Slaughter Association. 2018. Humane slaughter of finfish farmed around the world. Humane Slaughter Association.
83 Teletchea, Fabrice, and Pascal Fontaine. 2012. Levels of domestication in fish: implications for the sustainable future of aquaculture. Fish and Fisheries 15: 181–195. https://doi.org/10.1111/faf.12006.
84 Engel, Sandy. 1976. Food Habits and Prey Selection of Coho Salmon (Oncorhynchus kisutch) and Cisco (Coregonus artedii) in Relation to Zooplankton Dynamics in Pallette Lake, Wisconsin. Transactions of the American Fisheries Society 105: 607–614. https://doi.org/10.1577/1548-8659(1976)105<607:FHAPSO>2.0.CO;2.
85 Brodeur, Richard D., Robert C. Francis, and William G. Pearcy. 1992. Food Consumption of Juvenile Coho (Oncorhynchus kisutch) and Chinook Salmon (O. tshawytscha) on the Continental Shelf off Washington and Oregon. Canadian Journal of Fisheries and Aquatic Sciences 49: 1670–1685. Ottawa, Canada. https://doi.org/10.1139/f92-186.
86 Brodeur, Richard D., Elizabeth A. Daly, Robert A. Schabetsberger, and Kathryn L. Mier. 2007. Interannual and interdecadal variability in juvenile coho salmon (Oncorhynchus kisutch) diets in relation to environmental changes in the northern California Current. Fisheries Oceanography 16: 395–408. https://doi.org/https://doi.org/10.1111/j.1365-2419.2007.00438.x.
87 Sweeting, R M, and R J Beamish. 2009. A Comparison of the Diets of Hatchery and Wild Coho Salmon (Oncorhynchus kisutch) in the Strait of Georgia from 1997–2007. North Pacific Anadromous Fish Commission Bulletin: 255–264.
88 Arndt, Ronney E., Ronald W. Hardy, Shozo H. Sugiura, and Faye M. Dong. 1999. Effects of heat treatment and substitution level on palatability and nutritional value of soy defatted flour in feeds for Coho Salmon, Oncorhynchus kisutch. Aquaculture 180: 129–145. https://doi.org/10.1016/S0044-8486(99)00186-6.
89 Mahnken, Conrad V. W., John Spinelli, and F. William Waknitz. 1980. Evaluation of an alkane yeast (Candida sp.) as a substitute for fish meal in Oregon Moist Pellet: Feeding trials with coho salmon (Oncorhynchus kisutch) and rainbow trout (Salmo gairdneri). Aquaculture 20: 41–56. https://doi.org/10.1016/0044-8486(80)90060-5.
90 Yu, T. C., and R. O. Sinnhuber. 2011. Use of Beef Tallow as an Energy Source in Coho Salmon (Oncorhynchus kisutch) Rations. Canadian Journal of Fisheries and Aquatic Sciences 38: 367–370. Ottawa, Canada. https://doi.org/10.1139/f81-049.
91 Dosanjh, Bakhshish S., David A. Higgs, M. Dianne Plotnikoff, Jack R. McBride, Jack R. Markert, and J. T. Buckley. 1984. Efficacy of canola oil, pork lard and marine oil singly and in combination as supplemental dietary lipid sources for juvenile coho salmon (Oncorhynchus kisutch). Aquaculture 36: 333–345. https://doi.org/10.1016/0044-8486(84)90326-0.