Information
Version: B | 1.1 (2022-01-22)
Please note: This part of the profile is currently being revised.
WelfareScore | farm
Condensed assessment of the species' likelihood and potential for good fish welfare in aquaculture, based on ethological findings for 10 crucial criteria.
- Li = Likelihood that the individuals of the species experience good welfare under minimal farming conditions
- Po = Potential of the individuals of the species to experience good welfare under high-standard farming conditions
- Ce = Certainty of our findings in Likelihood and Potential
WelfareScore = Sum of criteria scoring "High" (max. 10)
General remarks
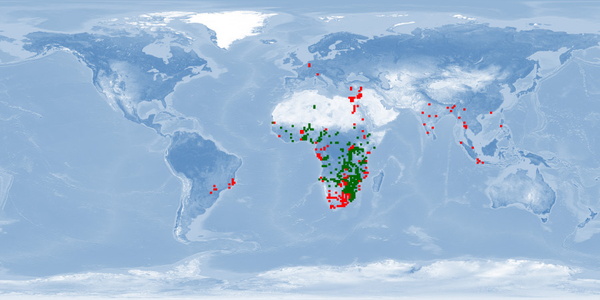
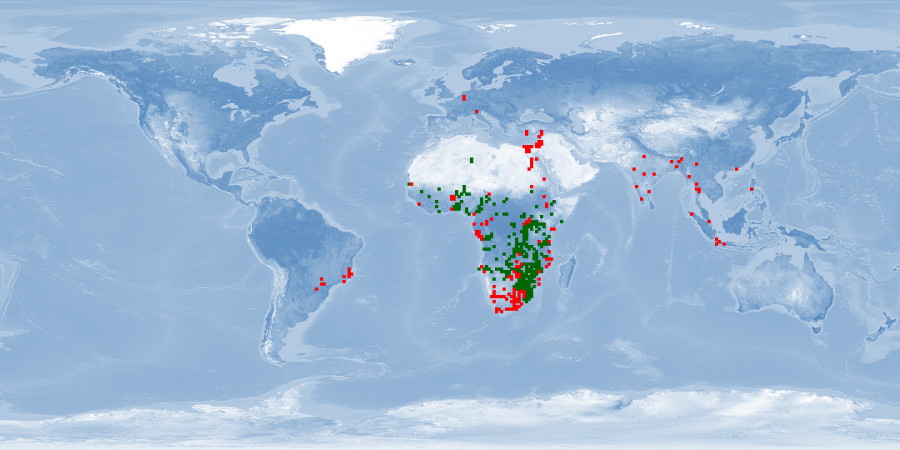
Clarias gariepinus is a very resilient and fast growing air-breathing fish. It is farmed mostly in Africa, South America, Asia and China, using extensive and traditional methods as well as intensive and industrial techniques. Probably due to its resilience, and despite promising biological traits, some aspects of welfare are either disregarded or overlooked. It is an aggressive fish and easily stressed by usual rearing conditions and practices. It is sensitive to environmental cues when spawning, and usual farming procedures for reproduction are highly invasive. If proper attention is given to these aspects and if reared in conditions that respect spatial needs, it has potential to be reared in good welfare conditions.
1 Home range
Many species traverse in a limited horizontal space (even if just for a certain period of time per year); the home range may be described as a species' understanding of its environment (i.e., its cognitive map) for the most important resources it needs access to.
What is the probability of providing the species' whole home range in captivity?
It is low for minimal farming conditions. It is high for high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


2 Depth range
Given the availability of resources (food, shelter) or the need to avoid predators, species spend their time within a certain depth range.
What is the probability of providing the species' whole depth range in captivity?
It is low for minimal farming conditions. It is high for high-standard farming conditions. Our conclusion is based on a high amount of evidence.


3 Migration
Some species undergo seasonal changes of environments for different purposes (feeding, spawning, etc.), and to move there, they migrate for more or less extensive distances.
What is the probability of providing farming conditions that are compatible with the migrating or habitat-changing behaviour of the species?
It is low for minimal farming conditions. It is high for high-standard farming conditions. Our conclusion is based on a high amount of evidence.


4 Reproduction
A species reproduces at a certain age, season, and sex ratio and possibly involving courtship rituals.
What is the probability of the species reproducing naturally in captivity without manipulation of theses circumstances?
It is low for minimal farming conditions. It is high for high-standard farming conditions. Our conclusion is based on a high amount of evidence.


5 Aggregation
Species differ in the way they co-exist with conspecifics or other species from being solitary to aggregating unstructured, casually roaming in shoals or closely coordinating in schools of varying densities.
What is the probability of providing farming conditions that are compatible with the aggregation behaviour of the species?
It is unclear for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


6 Aggression
There is a range of adverse reactions in species, spanning from being relatively indifferent towards others to defending valuable resources (e.g., food, territory, mates) to actively attacking opponents.
What is the probability of the species being non-aggressive and non-territorial in captivity?
It is low for minimal and high-standard farming conditions. Our conclusion is based on a low amount of evidence.


7 Substrate
Depending on where in the water column the species lives, it differs in interacting with or relying on various substrates for feeding or covering purposes (e.g., plants, rocks and stones, sand and mud, turbidity).
What is the probability of providing the species' substrate and shelter needs in captivity?
It is low for minimal farming conditions. It is high for high-standard farming conditions. Our conclusion is based on a high amount of evidence.


8 Stress
Farming involves subjecting the species to diverse procedures (e.g., handling, air exposure, short-term confinement, short-term crowding, transport), sudden parameter changes or repeated disturbances (e.g., husbandry, size-grading).
What is the probability of the species not being stressed?
It is low for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


9 Malformations
Deformities that – in contrast to diseases – are commonly irreversible may indicate sub-optimal rearing conditions (e.g., mechanical stress during hatching and rearing, environmental factors unless mentioned in crit. 3, aquatic pollutants, nutritional deficiencies) or a general incompatibility of the species with being farmed.
What is the probability of the species being malformed rarely?
It is low for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


10 Slaughter
The cornerstone for a humane treatment is that slaughter a) immediately follows stunning (i.e., while the individual is unconscious), b) happens according to a clear and reproducible set of instructions verified under farming conditions, and c) avoids pain, suffering, and distress.
What is the probability of the species being slaughtered according to a humane slaughter protocol?
It is unclear for minimal farming conditions. It is high for high-standard farming conditions. Our conclusion is based on a high amount of evidence.


Side note: Domestication
Teletchea and Fontaine introduced 5 domestication levels illustrating how far species are from having their life cycle closed in captivity without wild input, how long they have been reared in captivity, and whether breeding programmes are in place.
What is the species’ domestication level?
DOMESTICATION LEVEL 4 28, level 5 being fully domesticated.
Side note: Forage fish in the feed
450-1,000 milliard wild-caught fishes end up being processed into fish meal and fish oil each year which contributes to overfishing and represents enormous suffering. There is a broad range of feeding types within species reared in captivity.
To what degree may fish meal and fish oil based on forage fish be replaced by non-forage fishery components (e.g., poultry blood meal) or sustainable sources (e.g., soybean cake)?
All age classes: WILD: mostly carnivorous 1 2 9. FARM: fish meal and fish oil may be completely* replaced by sustainable sources 29 30 31.
*partly = <51% – mostly = 51-99% – completely = 100%
Glossary
12D = 12 h dark
12L = 12 h light
18D = 18 h dark
18L = 18 h light
24D = 24 h dark
6D = 6 h dark
6L = 6 h light
ADULTS = mature individuals, for details ➝ Findings 10.1 Ontogenetic development
DOMESTICATION LEVEL 4 = entire life cycle closed in captivity without wild inputs 28
FARM = setting in farming environment or under conditions simulating farming environment in terms of size of facility or number of individuals
FINGERLINGS = early juveniles with fully developed scales and working fins, the size of a human finger; for details ➝ Findings 10.1 Ontogentic development
IND = individuals
JUVENILES = fully developed but immature individuals, for details ➝ Findings 10.1 Ontogenetic development
LARVAE = hatching to mouth opening, for details ➝ Findings 10.1 Ontogenetic development
PHOTOPERIOD = duration of daylight
SPAWNERS = adults during the spawning season; in farms: adults that are kept as broodstock
WILD = setting in the wild
Bibliography
2 Bruton, M. N. 1979. The breeding biology and early development of Clarias gariepinus (Pisces: Clariidae) in Lake Sibaya, South Africa, with a review of breeding in species of the subgenus Clarias (Clarias). The Transactions of the Zoological Society of London 35: 1–45. https://doi.org/10.1111/j.1096-3642.1979.tb00056.x.
3 de Graaf, Gertjan, and Johannes Janssen. 1996. Handbook on the artificial reproduction and pond rearing of the African catfish Clarias gariepinus in sub-Saharan Africa. FAO Fisheries Technical Paper 362. Rome: Food and Agriculture Organization of the United Nations.
4 Akinwole, A. O., and E. O. Faturoti. 2007. Biological performance of African Catfish (Clarias gariepinus) cultured in recirculating system in Ibadan. Aquacultural Engineering 36: 18–23. https://doi.org/10.1016/j.aquaeng.2006.05.001.
5 Hocutt, Charles H. 1989. Seasonal and diel behaviour of radio-tagged Clarias gariepinus in Lake Ngezi, Zimbabwe (Pisces: Clariidae). Journal of Zoology 219: 181–199. https://doi.org/10.1111/j.1469-7998.1989.tb02575.x.
6 Pouomogne, V. 2010. Cultured Aquatic Species Information Programme. Clarias gariepinus. Rome: FAO Fisheries and Aquaculture Department.
7 Toko, Imorou, Emile D. Fiogbe, Bruno Koukpode, and Patrick Kestemont. 2007. Rearing of African catfish (Clarias gariepinus) and vundu catfish (Heterobranchus longifilis) in traditional fish ponds (whedos): Effect of stocking density on growth, production and body composition. Aquaculture 262: 65–72. https://doi.org/10.1016/j.aquaculture.2006.08.054.
8 El Naggar, Gamal O., George John, Mahmoud A. Rezk, Waheed Elwan, and Mohammed Yehia. 2006. Effect of varying density and water level on the spawning response of African catfish Clarias gariepinus: Implications for seed production. Aquaculture 261: 904–907. https://doi.org/10.1016/j.aquaculture.2006.07.043.
9 Dadebo, Elias. 2000. Reproductive biology and feeding habits of the catfish Clarias gariepinus (Burchell)(Pisces: Clariidae) in Lake Awassa, Ethiopia. SINET: Ethiopian Journal of Science 23: 231–246.
10 Almazán-Rueda, P., A. T. M Van Helmond, J. a. J. Verreth, and J. W. Schrama. 2005. Photoperiod affects growth, behaviour and stress variables in Clarias gariepinus. Journal of Fish Biology 67: 1029–1039. https://doi.org/10.1111/j.0022-1112.2005.00806.x.
11 van der Waal, B. C. W. 1974. Observations on the breeding habits of Clarias gariepinus (Burchell). Journal of Fish Biology 6: 23–27. https://doi.org/10.1111/j.1095-8649.1974.tb04518.x.
12 Huisman, E. A., and C. J. J. Richter. 1987. Reproduction, growth, health control and aquacultural potential of the African catfish, Clarias gariepinus (Burchell 1822). Aquaculture 63. Culture of Clarias Species: 1–14. https://doi.org/10.1016/0044-8486(87)90057-3.
13 de Graaf, G J, F Galemoni, and B Banzoussi. 1995. Artificial reproduction and fingerling production of the African catfish, Clarias gariepinus (Burchell 1822), in protected and unprotected ponds. Aquaculture Research 26: 233–242. https://doi.org/10.1111/j.1365-2109.1995.tb00908.x.
14 NOT FOUND
15 Almazán-Rueda, Pablo, Johan W Schrama, and Johan A. J Verreth. 2004. Behavioural responses under different feeding methods and light regimes of the African catfish (Clarias gariepinus) juveniles. Aquaculture 231: 347–359. https://doi.org/10.1016/j.aquaculture.2003.11.016.
16 Adewolu, Morenike A., Comfort A. Adeniji, and Ademola B. Adejobi. 2008. Feed utilization, growth and survival of Clarias gariepinus (Burchell 1822) fingerlings cultured under different photoperiods. Aquaculture 283: 64–67. https://doi.org/10.1016/j.aquaculture.2008.07.020.
17 Van Wassenbergh, Sam, Anthony Herrel, Dominique Adriaens, and Peter Aerts. 2005. A test of mouth-opening and hyoid-depression mechanisms during prey capture in a catfish using high-speed cineradiography. The Journal of Experimental Biology 208: 4627–4639. https://doi.org/10.1242/jeb.01919.
18 Van Wassenbergh, Sam, Tim Lieben, Anthony Herrel, Frank Huysentruyt, Tom Geerinckx, Dominique Adriaens, and Peter Aerts. 2009. Kinematics of benthic suction feeding in Callichthyidae and Mochokidae, with functional implications for the evolution of food scraping in catfishes. Journal of Experimental Biology 212: 116–125. https://doi.org/10.1242/jeb.023929.
19 Haylor, G. S. 1992. Controlled hatchery production of Clarias gariepinus (Burchell): growth and survival of larvae at high stocking density. Aquaculture Research 23: 303–314. https://doi.org/10.1111/j.1365-2109.1992.tb00773.x.
20 Adeyemo, Olanike K, Irene Naigaga, and Rashidat A Alli. 2009. Effect of handling and transportation on haematology of African catfish (Clarias gariepinus). Journal of FisheriesSciences. com 3: 333–341.
21 van de Nieuwegiessen, Pascal G., Annette S. Boerlage, Johan A. J. Verreth, and Johan W. Schrama. 2008. Assessing the effects of a chronic stressor, stocking density, on welfare indicators of juvenile African catfish, Clarias gariepinus Burchell. Applied Animal Behaviour Science 115: 233–243. https://doi.org/10.1016/j.applanim.2008.05.008.
22 Adebayo, OT. 2006. Reproductive performance of African Clariid Catfish Clarias gariepinus broodstock on varying maternal stress. Journal of Fisheries international 1: 17–20.
23 Eya, Jonathan C. 1996. ‘Broken-Skull Disease’ in African Catfish Clarias gariepinus is Related to a Dietary Deficiency of Ascorbic Acid. Journal of the World Aquaculture Society 27: 493–498. https://doi.org/10.1111/j.1749-7345.1996.tb00635.x.
24 Viveen, W. J. A. R., C. J. J. Richter, P. G. W. J. Van Oordt, J. A. L. Janssen, and E. A. Huisman. 1985. Practical manual for the culture of the African catfish (Clarias gariepinus).
25 Lambooij, E, R J Kloosterboer, C Pieterse, M A Gerritzen, and J W Van de vis. 2003. Stunning of farmed African catfish (Clarias gariepinus) using a captive needle pistol; assessment of welfare aspects. Aquaculture Research 34: 1353–1358. https://doi.org/10.1046/j.1365-2109.2003.00966.x.
26 Sattari, A., E. Lambooij, H. Sharifi, W. Abbink, H. Reimert, and J. W. van de Vis. 2010. Industrial dry electro-stunning followed by chilling and decapitation as a slaughter method in Claresse® (Heteroclarias sp.) and African catfish (Clarias gariepinus). Aquaculture 302: 100–105. https://doi.org/10.1016/j.aquaculture.2010.01.011.
27 Lambooij, E., R. J. Kloosterboer, M. A. Gerritzen, and J. W. van de Vis. 2006. Assessment of electrical stunning in fresh water of African Catfish (Clarias gariepinus) and chilling in ice water for loss of consciousness and sensibility. Aquaculture 254: 388–395. https://doi.org/10.1016/j.aquaculture.2005.10.027.
28 Teletchea, Fabrice, and Pascal Fontaine. 2012. Levels of domestication in fish: implications for the sustainable future of aquaculture. Fish and Fisheries 15: 181–195. https://doi.org/10.1111/faf.12006.
29 Fasakin, E A, A M Balogun, and O O Ajayi. 2003. Evaluation of full-fat and defatted maggot meals in the feeding of clariid catfish Clarias gariepinus fingerlings. Aquaculture Research 34: 733–738. https://doi.org/10.1046/j.1365-2109.2003.00876.x.
30 Sotolu, AO, and others. 2009. Comparative utilizations of fish waste meal with imported fishmeal by African Catfish (Clarias gariepinus). American-Euras J. Sci. Res 4: 285–289.
31 Nyina-Wamwiza, L., B. Wathelet, J. Richir, X. Rollin, and P. Kestemont. 2010. Partial or total replacement of fish meal by local agricultural by-products in diets of juvenile African catfish (Clarias gariepinus): growth performance, feed efficiency and digestibility. Aquaculture Nutrition 16: 237–247. https://doi.org/10.1111/j.1365-2095.2009.00658.x.