Information
Version: B | 1.1 (2022-06-23)
WelfareScore | farm
Condensed assessment of the species' likelihood and potential for good fish welfare in aquaculture, based on ethological findings for 10 crucial criteria.
- Li = Likelihood that the individuals of the species experience good welfare under minimal farming conditions
- Po = Potential of the individuals of the species to experience good welfare under high-standard farming conditions
- Ce = Certainty of our findings in Likelihood and Potential
WelfareScore = Sum of criteria scoring "High" (max. 10)
General remarks
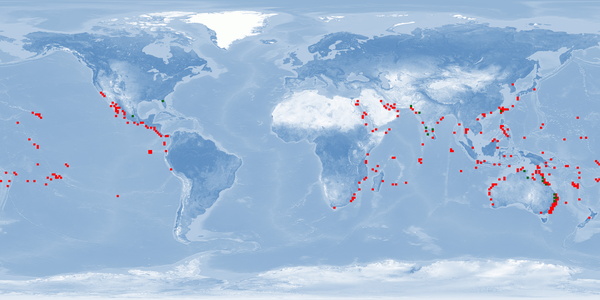
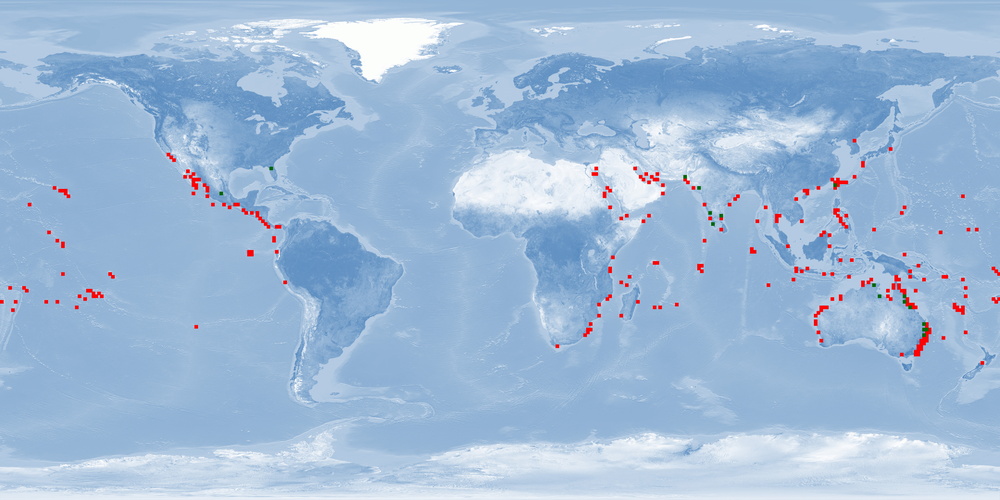
Chanos chanos is a BENTHOPELAGIC Indo-Pacific fish species that is found along continental shelves and around islands of low latitude tropics or in the subtropical northern hemisphere where temperatures are higher than 20 °C. This euryhaline fish can be found in fresh, brackish, and marine waters, occurring in small to large schools near the coasts or around islands where reefs are well developed. It is an AMPHIDROMOUS species: SPAWNERS release eggs in oceanic waters, then older larvae migrate onshore and settle in coastal wetlands like mangroves or estuaries (occasionally entering freshwater lakes), and JUVENILES then migrate back to sea where they mature sexually. C. chanos is especially valued as a food fish in Southeast Asia and also used in game fish as bait. Taiwan, Indonesia, and Philippines – countries that started to culture this fish about 4-6 centuries ago – are the main producers. This fish is tolerant to low concentrations of oxygen and is usually farmed in ponds, pens or cages in wide salinity ranges. Although being able to spawn naturally in captivity, the traditional farming usually depended on an annual restocking of ponds with FINGERLINGS reared from wild-caught FRY. Now farms are obtaining FRY from hatcheries. C. chanos is harvested and marketed mostly fresh or chilled, whole or deboned, frozen or processed. When harvested, individuals are JUVENILES. Thus, farming information about ADULTS are usually restricted to broodstock. Moreover, further studies about home range, aggression, substrate availability in farms, and stunning and slaughtering protocols are still needed for this species.
1 Home range
Many species traverse in a limited horizontal space (even if just for a certain period of time per year); the home range may be described as a species' understanding of its environment (i.e., its cognitive map) for the most important resources it needs access to.
What is the probability of providing the species' whole home range in captivity?
It is unclear for minimal and high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


2 Depth range
Given the availability of resources (food, shelter) or the need to avoid predators, species spend their time within a certain depth range.
What is the probability of providing the species' whole depth range in captivity?
It is low for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


3 Migration
Some species undergo seasonal changes of environments for different purposes (feeding, spawning, etc.), and to move there, they migrate for more or less extensive distances.
What is the probability of providing farming conditions that are compatible with the migrating or habitat-changing behaviour of the species?
It is low for minimal and high-standard farming conditions. Our conclusion is based on a high amount of evidence.


4 Reproduction
A species reproduces at a certain age, season, and sex ratio and possibly involving courtship rituals.
What is the probability of the species reproducing naturally in captivity without manipulation of theses circumstances?
It is low for minimal farming conditions. It is high for high-standard farming conditions. Our conclusion is based on a high amount of evidence.


5 Aggregation
Species differ in the way they co-exist with conspecifics or other species from being solitary to aggregating unstructured, casually roaming in shoals or closely coordinating in schools of varying densities.
What is the probability of providing farming conditions that are compatible with the aggregation behaviour of the species?
It is unclear for minimal and high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


6 Aggression
There is a range of adverse reactions in species, spanning from being relatively indifferent towards others to defending valuable resources (e.g., food, territory, mates) to actively attacking opponents.
What is the probability of the species being non-aggressive and non-territorial in captivity?
It is low for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a low amount of evidence.


7 Substrate
Depending on where in the water column the species lives, it differs in interacting with or relying on various substrates for feeding or covering purposes (e.g., plants, rocks and stones, sand and mud, turbidity).
What is the probability of providing the species' substrate and shelter needs in captivity?
It is low for minimal farming conditions. It is high for high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


8 Stress
Farming involves subjecting the species to diverse procedures (e.g., handling, air exposure, short-term confinement, short-term crowding, transport), sudden parameter changes or repeated disturbances (e.g., husbandry, size-grading).
What is the probability of the species not being stressed?
It is low for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a high amount of evidence.


9 Malformations
Deformities that – in contrast to diseases – are commonly irreversible may indicate sub-optimal rearing conditions (e.g., mechanical stress during hatching and rearing, environmental factors unless mentioned in crit. 3, aquatic pollutants, nutritional deficiencies) or a general incompatibility of the species with being farmed.
What is the probability of the species being malformed rarely?
It is low for minimal and high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


10 Slaughter
The cornerstone for a humane treatment is that slaughter a) immediately follows stunning (i.e., while the individual is unconscious), b) happens according to a clear and reproducible set of instructions verified under farming conditions, and c) avoids pain, suffering, and distress.
What is the probability of the species being slaughtered according to a humane slaughter protocol?
There are no findings for minimal and high-standard farming conditions.


Side note: Domestication
Teletchea and Fontaine introduced 5 domestication levels illustrating how far species are from having their life cycle closed in captivity without wild input, how long they have been reared in captivity, and whether breeding programmes are in place.
What is the species’ domestication level?
DOMESTICATION LEVEL 4 41, level 5 being fully domesticated.
Side note: Forage fish in the feed
450-1,000 milliard wild-caught fishes end up being processed into fish meal and fish oil each year which contributes to overfishing and represents enormous suffering. There is a broad range of feeding types within species reared in captivity.
To what degree may fish meal and fish oil based on forage fish be replaced by non-forage fishery components (e.g., poultry blood meal) or sustainable sources (e.g., soybean cake)?
WILD: omnivorous 24 29 25 1 42, mainly feeding on blue-green algae 11, but FRY and JUVENILES also on detritus and plant materials 2 and FRY on plankton 24 11 1. FARM: fertilised ponds with no supplementary feeding is possible 24 14 15 33, but supplemental feeding (e.g., rice bran or copra meal) is used for better growth 24 14. Fish meal may not be replaced in FRY 9, but may be completely* replaced by sustainable resources for JUVENILES 17. LAB: FRY: fish meal may be not 38 to partly* 43 44 replaced by sustainable or non-forage fishery sources; fish oil may be partly* 44 to completely* replaced by sustainable sources, with better growth and survival when mostly* replaced 40. JUVENILES: fish meal may be partly* replaced by sustainable sources 42.
partly = <51% – mostly = 51-99% – completely = 100%
Glossary
AMPHIDROMOUS = migrating between fresh water and sea independent of spawning
BENTHIC = living at the bottom of a body of water, able to rest on the floor
BENTHOPELAGIC = living and feeding near the bottom of a body of water, floating above the floor
DOMESTICATION LEVEL 4 = entire life cycle closed in captivity without wild inputs 41
EURYHALINE = tolerant of a wide range of salinities
FARM = setting in farming environment or under conditions simulating farming environment in terms of size of facility or number of individuals
FINGERLINGS = early juveniles with fully developed scales and working fins, the size of a human finger; for details ➝ Findings 10.1 Ontogentic development
FRY = larvae from external feeding on, for details ➝ Findings 10.1 Ontogenetic development
IND = individuals
JUVENILES = fully developed but immature individuals, for details ➝ Findings 10.1 Ontogenetic development
LAB = setting in laboratory environment
LARVAE = hatching to mouth opening, for details ➝ Findings 10.1 Ontogenetic development
PELAGIC = living independent of bottom and shore of a body of water
PHOTOPERIOD = duration of daylight
PLANKTONIC = horizontal movement limited to hydrodynamic displacement
SPAWNERS = adults during the spawning season; in farms: adults that are kept as broodstock
WILD = setting in the wild
Bibliography
2 Kumagai, S., T. Bagarinao, and A. Unggui. 1985. Growth of juvenile milkfish Chanos chanos in a natural habitat. Marine Ecology Progress Series 22: 1–6.
3 Lalramchhani, C., C. P. Balasubramanian, A. Panigrahi, T. K. Ghoshal, S. Das, P. S. S. Anand, and K. K. Vijayan. 2019. Polyculture of Indian White Shrimp (Penaeus indicus) with Milkfish (Chanos chanos) and its Effect on Growth Performances, Water Quality and Microbial Load in Brackishwater Pond. Journal of Coastal Research 86: 43–48. https://doi.org/10.2112/SI86-006.1.
4 Prijono, A., Tridjoko, I. N. A. Giri, A. Poernomo, W. E. Vanstone, C. Lim, and T. Daulay. 1988. Natural spawning and larval rearing of milkfish in captivity in Indonesia. Aquaculture 74: 127–130.
5 Emata, A. C., and C. L. Marte. 1994. Natural spawning, egg and fry production of milkfish, Chanos chanos (Forsskal), broodstock reared in concrete tanks1. Journal of Applied Ichthyology 10: 10–16. https://doi.org/10.1111/j.1439-0426.1994.tb00139.x.
6 Juario, J. V., M. N. Duray, V. M. Duray, J. F. Nacario, and J. M. E. Almendras. 1984. Induced breeding and larval rearing experiments with milkfish Chanos chanos (Forskal) in the Philippines. Aquaculture 36: 61–70. https://doi.org/10.1016/0044-8486(84)90054-1.
7 Marte, C. L., and F. J. Lacanilao. 1986. Spontaneous maturation and spawning of milkfish in floating net cages. Aquaculture 53: 115–132. https://doi.org/10.1016/0044-8486(86)90281-4.
8 Baliao, D. D., E. M. Rodriguez, and D. D. Gerochi. 1980. Growth and survival rates of hatchery-produced and wild milkfish fry grown to fingerling size in earthen nursery ponds. SEAFDEC Aquaculture Department Quarterly Research Report 4: 11–14.
9 Santiago, Corazon B., Julia B. Pantastico, Susana F. Baldia, and Ofelia S. Reyes. 1989. Milkfish (Chanos chanos) fingerling production in freshwater ponds with the use of natural and artificial feeds. Aquaculture 77: 307–318. https://doi.org/10.1016/0044-8486(89)90215-9.
10 Hilomen-Garcia, G. V. 1997. Morphological abnormalities in hatchery-bred milkfish (Chanos chanos Forsskal) fry and juveniles. Aquaculture 152: 155–166. https://doi.org/10.1016/S0044-8486(96)01518-9.
11 Cruz, E. M., and I. L. Laudencia. 1980. Polyculture of milkfish (Chanos chanos Forskal), all-male Nile tilapia (Tilapia nilotica) and snakehead (Ophicephalus striatus) in freshwater ponds with supplemental feeding. Aquaculture 20: 231–237. https://doi.org/10.1016/0044-8486(80)90113-1.
12 Pinto, L. 1982. Growth of Chanos chanos Forskal and Tilapia mossambica in a fish pond-saltern complex in Las Pinas, The Philippines. Aquaculture 29: 169–170.
13 Sumagaysay-Chavoso, N. S., and M. L. San Diego-McGlone. 2003. Water quality and holding capacity of intensive and semi-intensive milkfish (Chanos chanos) ponds. Aquaculture 219: 413–429. https://doi.org/10.1016/S0044-8486(02)00576-8.
14 Biswas, G., J. K. Sundaray, A. R. Thirunavukkarasu, and M. Kailasam. 2011. Length-weight relationship and variation in condition of Chanos chanos (Forsskål, 1775) from tide-fed brackishwater ponds of the Sunderbans - India. Indian Journal of Geo-Marine Science 40: 386–390.
15 Mirera, D. O. 2011. Experimental Polyculture of Milkfish (Chanos chanos) and Mullet (Mugil cephalus) Using Earthen Ponds. Western Indian Ocean Journal of Marine Science 10: 59–71. https://doi.org/10.4314/WIOJMS.V10I1.
16 Hanke, I., C. Hassenrück, B. Ampe, A. Kunzmann, A. Gärdes, and J. Aerts. 2020. Chronic stress under commercial aquaculture conditions: Scale cortisol to identify and quantify potential stressors in milkfish (Chanos chanos) mariculture. Aquaculture 526: 735352. https://doi.org/10.1016/j.aquaculture.2020.735352.
17 NOT FOUND
18 Emata, A. C. 2000. Live Transport of Pond‐reared Milkfish Chanos chanos Broodstock. Journal of the World Aquaculture Society 31: 279–282. https://doi.org/10.1111/j.1749-7345.2000.tb00364.x.
19 Marte, C. L., N. M. Sherwood, L. W. Crim, and B. Harvey. 1987. Induced spawning of maturing milkfish (Chanos chanos Forsskal) with gonadotropin-releasing hormone (GnRH) analogues administered in various ways. Aquaculture 60: 303–310. https://doi.org/10.1016/0044-8486(87)90295-X.
20 Marte, C. L., N. Sherwood, L. Crim, and J. Tan. 1988. Induced spawning of maturing milkfish (Chanos chanos) using human chorionic gonadotropin and mammalian and salmon gonadotropin releasing hormone analogues. Aquaculture 73: 333–340. https://doi.org/10.1016/0044-8486(88)90066-X.
21 Garcia, L. M. B., G. V. Hilomen-Garcia, and A. C. Emata. 2000. Survival of captive milkfish Chanos chanos Forsskal broodstock subjected to handling and transport. Aquaculture Research 31: 575–583. https://doi.org/10.1046/j.1365-2109.2000.00475.x.
22 Lee, C.-S., C. S. Tamaru, C. D. Kelley, and J. E. Banno. 1986. Induced spawning of milkfish, Chanos chanos, by a single application of LHRH-analogue. Aquaculture 58: 87–98. https://doi.org/10.1016/0044-8486(86)90158-4.
23 Toledo, J. D., and A. G. Gaitan. 1992. Egg cannibalism by milkfish (Chanos chanos Forsskal) spawners in circular floating net cages. Journal of Applied Ichthyology 8: 257–262. https://doi.org/10.1111/j.1439-0426.1992.tb00692.x.
24 Froese, R., and D. Pauly. 2022. Chanos chanos, Milkfish: fisheries, aquaculture, aquarium. World Wide Web electronic publication. FishBase. https://www.fishbase.se/summary/citation.php. Accessed January 13.
25 Bagarinao, T. U. 1991. BIOLOGY OF MILKFISH (Chanos chanos Forsskal). Tigbauan, Iloilo, Philippines: Aquaculturc Department Southeast Asian Fisheries Development Center (SEAFDEC).
26 Bagarinao, T., and S. Kumagai. 1987. Occurrence and distribution of milkfish larvae,Chanos chanos off the western coast of Panay Island, Philippines. Environmental Biology of Fishes 19: 155–160. https://doi.org/10.1007/BF00001886.
27 NOT FOUND
28 Bacchet, P., T. Zysman, and Y. Lefèvre. 2006. Guide des poissons de Tahiti et ses îles. Tahiti (Polynésie Francaise): Éditions Au Vent des Îles.
29 Bagarinao, T., and K. Thayaparan. 1986. The length-weight relationship, food habits and condition factor of wild juvenile milkfish in Sri Lanka. Aquaculture 55: 241–246. https://doi.org/10.1016/0044-8486(86)90119-5.
30 Martosudarmo, B., S. Noor-Hamid, and S. Sabaruddin. 1976. Occurrence of milkfish, Chanos chanos (Forsskal), spawners in the Karimun Jawa waters. Bull. Shrimp Cult. Res. Centre Jepara 2: 169–176.
31 Riede, K. 2004. Global register of migratory species - from global to regional scales. Final report of the R&D Projekt 808 05 081. Bonn, Germany: Federal Agency for Nature Conservation.
32 Shadrack, R. S., S. Gereva, T. Pickering, and M. Ferreira. 2021. Seasonality, abundance and spawning season of milkfish Chanos chanos (Forsskål, 1775) at Teouma Bay, Vanuatu. Marine Policy 130: 104587. https://doi.org/10.1016/j.marpol.2021.104587.
33 A’yun, Q., and N. D. Takarina. 2019. Food preference analysis of milkfish in Blanakan Ponds, Subang, West Java. AIP Conference Proceedings 2168: 020083. https://doi.org/10.1063/1.5132510.
34 Kanashiro, K., and S. Asato. 1985. Mature Milkfish, Chanos chanos, Caught in Okinawa Island, Japan. Japanese Journal of Ichthyology 31: 434–437. https://doi.org/10.11369/jji1950.31.434.
35 Kuo, C.-M., and C. E. Nash. 1979. Annual reproductive cycle of milkfish, Chanos chanos Forskal, in Hawaiian waters. Aquaculture 16: 247–251. https://doi.org/10.1016/0044-8486(79)90113-3.
36 Rabanal, H. R., R. S. Esguerra, and M. M. Nepomuceno. 1953. Studies on the rate of growth of milkfish, Chanos chanos Forsskal, under cultivation. I. Rate of growth of fry and fingerlings in fishpond nurseries. Proc. Indo-Pac. Fish. Coun. 4: 171–180.
37 Villegas, C. T., and I. Bombeo. 1981. Effects of increased stocking density and supplemental feeding on the production of milkfish fingerlings. SEAFDEC Aquaculture Dept. Quart. Res. Rep. 5: 7–ll.
38 Seneriches, M. L. M., and Y. N. Chiu. 1988. Effect of fishmeal on the growth, survival and feed efficiency of milkfish (Chanos chanos) fry. Aquaculture 71: 61–69. https://doi.org/10.1016/0044-8486(88)90273-6.
39 Bhaskar, B., and K. S. Srinivasa. 1989. Influence of environmental variables on haematology, and compendium of normal haematological ranges of milkfish, Chanos chanos (Forskal), in brackishwater culture. Aquaculture 83: 123–136. https://doi.org/10.1016/0044-8486(89)90066-5.
40 Sivaramakrishnan, T., K. Ambasankar, K. P. K. Vasagam, J. S. Dayal, K. P. Sandeep, A. Bera, M. Makesh, M. Kailasam, and K. K. Vijayan. 2021. Effect of dietary soy lecithin inclusion levels on growth, feed utilization, fatty acid profile, deformity and survival of milkfish (Chanos chanos) larvae. Aquaculture Research 52: 5366–5374. https://doi.org/10.1111/are.15406.
41 Teletchea, Fabrice, and Pascal Fontaine. 2012. Levels of domestication in fish: implications for the sustainable future of aquaculture. Fish and Fisheries 15: 181–195. https://doi.org/10.1111/faf.12006.
42 Bharathi, S., C. Antony, C. B. T. Rajagopalsamy, A. Uma, B. Ahilan, R. S. S. Lingam, S. F. Khan, and E. Prabu. 2020. Partial replacement of fishmeal with soybean meal and distillers dried grain solubles (DDGS) as alternative protein sources for milkfish, Chanos chanos (Forsskal, 1775) fingerlings. Indian Journal of Fisheries 67: 62–70. https://doi.org/10.21077/ijf.2020.67.4.99940-08.
43 Santiago, C. B., M. Bañes-Aldaba, and E. T. Songalia. 1983. Effect of artificial diets on growth and survival of milkfish fry in fresh water. Aquaculture 34: 247–252. https://doi.org/10.1016/0044-8486(83)90206-5.
44 Alava, V. R., and C. Lim. 1988. Artificial diets for milkfish, Chanos chanos (Forsskal), fry reared in seawater. Aquaculture 71: 339–346. https://doi.org/10.1016/0044-8486(88)90203-7.